CENTERS FOR DIETARY SUPPLEMENT RESEARCH: BOTANICALS
RFA (OD-00-004)
Summary of the Applicant Information Meeting
January 28, 2000 (8:00 AM ñ 12:30 PM)
Executive Plaza North, 6130 Executive Boulevard
Rockville, MD
Purpose: To improve the quality of applications submitted in response to the Request for Applications (RFA), as well as to give potential applicants the opportunity to clarify any issues or questions concerning the RFA, an Applicant Information Meeting was held. Application concepts were briefly discussed. Outlines of individual proposals were not discussed with Program Staff.
Agenda
Introduction- Dr. Christine Swanson and Dr. Paul Coates
The Botanical Research Initiative-Dr. Swanson
The Review Process-Dr. Eugene Hayunga and Dr. Neal West
Grants Management- Ms. Suzanne White
Intellectual Property- Mr. Ted Roumel
Note: NIH staff attending the meeting are listed on the last page of this document.
INTRODUCTION
Dr. Swanson opened the meeting by stating the purpose of the AIM and introducing NIH staff. She distributed the agenda, indicating that NIH staff would make presentations to be followed by question and answer periods. (Questions arose before, during and after presentations. Questions asked by potential applicants are indicated in bold type.)
Dr. Coates welcomed the meeting participants and gave an overview of activities and responsibilities of the Office of Dietary Supplements (ODS). As part of its Congressional mandate, the ODS supports and coordinates NIH research activities in the area of dietary supplements. The RFA under discussion was developed as a collaborative effort involving several Institutes and Centers (ICs) at the NIH. The Dietary Supplement Research Centers Program is a major activity of the ODS. The ODS budget for FY 2000 includes funds for one new Dietary Supplement Research Center on Botanicals. The participating ICs may provide additional funding, depending on the quality of the grant applications received and IC funding priorities. Dr.Coates noted that as an Office within the NIH, the ODS does not have grant funding or grants management authority. These responsibilities are assumed by the ICs.
THE BOTANICAL RESEARCH INITIATIVE
Dr. Swanson described the development of the Dietary Supplement Research Centers Program. In FY 1999 the ODS received a funded Congressional mandate to "develop a botanical research initiative with major research institutions in the U.S." Congress allocated two million dollars for this activity. The ODS Strategic Plan calls for eight Dietary Supplement Research Centers, including four Centers devoted to the study of botanicals. The Congressional FY 1999 appropriation provided an opportunity to establish the first NIH-funded research center on botanicals. In collaboration with several ICs, the ODS developed and announced an RFA (OD-99-007) entitled Centers for Dietary Supplement Research: Botanicals. The RFA was released on March 8 with a receipt date of May 13, 1999. The Center for Scientific Review (CSR) convened a Special Emphasis Panel (SEP) to review the applications. The review took place in June of 1999. The results of the SEP review were presented in August to the Advisory Council of the National Center for Complementary and Alternative Medicine (NCCAM). NCCAM, the National Institute of General Medical Sciences (NIGMS) and the Office of Research on Womenís Health (ORWH) provided funding sufficient to support a second Center. The two awards were made and assigned to NCCAM for administrative purposes. NCCAM, NIGMS and the ORWH maintain a strong research interest in the two Centers. The National Institute of Environmental Health Sciences (NIEHS) expressed an interest in a third proposal which then was funded as a Program Project in FY 2000.
FY 99 Awards
One award was made to the University of Illinois at Chicago (UIC) with Dr. Norman Farnsworth as the PI. The UIC Center will focus its research efforts on herbal supplements that have implications for benefit in womenís health issues, including therapies for menopause. The UCLA Center, directed by Dr. David Heber will conduct research on herbs and other botanicals. Initially they will conduct research on St. Johnís Wort, green tea extract and yeast fermented rice. A NIH press release summarizing the activities of the two research Centers is provided at the following web site http://ods.od.nih.gov/News/NIH_Office_of_Dietary_Supplements_Announces_Funding_of_Dietary_Supplements_Research_Centers.aspx. Additional public information describing the research activities of the two Centers and the Program Project funded by NIEHS is provided in the NIH CRISP (Computer Retrieval of Information on Scientific Projects) database. The web link to CRISP is
https://www-commons.cit.nih.gov/crisp/. For P50 awards, CRISP provides a summary of the overall research proposal. Abstracts are not available through CRISP for individual research projects within the proposal.
Objectives of FY 2000 RFA
With continued Congressional support for the botanical initiative, the ODS intends to expand the program and fund additional research Centers on botanicals. One goal is to develop a critical mass of investigators focused on research in this area. The RFA under discussion is similar to the one issued in FY 1999. "The major goal of the RFA is to foster interdisciplinary research in order to promote the scientific study of botanicals, particularly those available as dietary supplements. Further, this RFA is intended to explore more fully the potential role of botanical dietary supplements as a significant part of the efforts of the United States to improve health care" (verbatim page 1 of the most recent RFA).
Several areas of emphasis are proposed under the RESEARCH OBJECTIVES section. In addition, each IC listed as a sponsor of the RFA provided a research interest statement.
The ODS can not provide guidance as to whether applicants should focus on specific areas of biological activity or specific health outcomes. Similarly, the ODS cannot provide guidance as to whether applicants should study a variety of botanicals or focus on a specific botanical. Those decisions are the responsibility of the PI and other investigators submitting the grant application.
As stated in the RFA, it is anticipated that one award will be made. Additional awards will depend on funding by the ICs listed on the RFA. As was the case in FY 1999, none of the ICs has made a financial commitment to the ODS Botanical Research Center Program for FY 2000. While none of the ICs is committed to individually funding a second Center or contributing funds for a second Center, the ODS could recommend a Center which focused on the research interest statements of a non-funding IC. For example, if the grant application with the lowest score (i.e., highest rank) focused on botanicals and cancer or on botanicals and digestive diseases, the ODS would be inclined to recommend funding based primarily on the priority score assigned by the SEP.
How do other ICs at NIH learn about the applications? Program Staff are aware of the research interests of the participating ICs. When the applications are received, they are read to identify proposals or research projects within proposals that may be of interest to particular ICs. Program Staff will encourage those ICs to read the applications and attend the SEP review. This process worked very well in FY99.
What is the definition of a botanical? The definition is provided in the last section of the RFA under definition of terms.
If a botanical in not currently available in the market place, can it be included in this RFA? Yes. The reviewers, however, will carefully evaluate the rationale behind the selection of the botanical(s) to be studied.
Should the Center research be focused on isolated chemical constituents derived from botanicals or on a less processed botanical such as a dried herb?
This is the decision of the PI and other investigators. The study of isolated constituents derived from botanicals will undoubtedly play an important role in botanical research. However, the isolation of active constituents for the primary purpose of drug development is not the intention of this RFA.
Suppose I want to do work on a specific phytochemical as it relates to a certain physiologic process. Why do I need a botany or plant science core to conduct this work? You donít. However, the fact that you do not need collaboration in the area of botany or plant science suggests that the research is not responsive the RFA. The research project(s) might be better suited to a regular R01 submission.
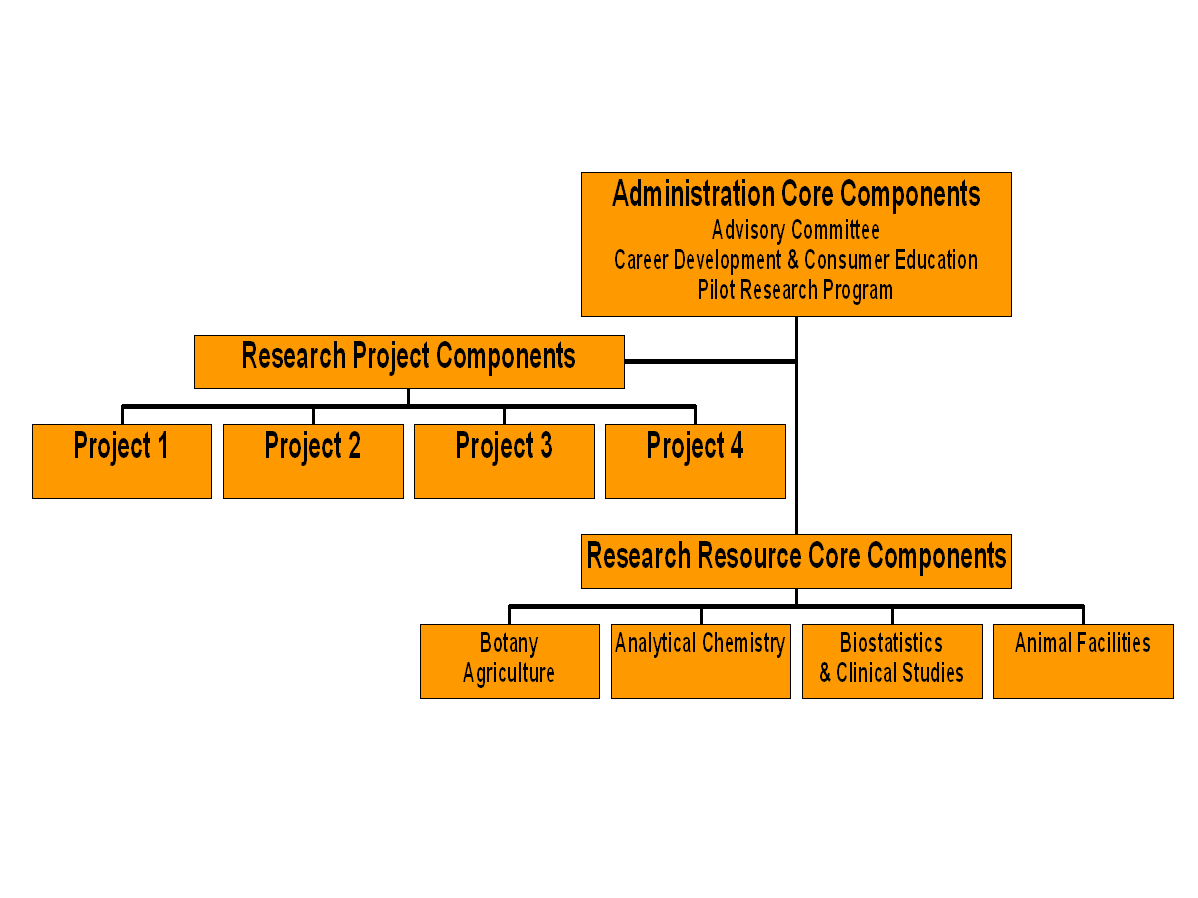
Center Structure and Requirements (see figure below)

Applicants must include two Cores and Research Projects in their applications. This figure is not intended to be used as a template in the grant applications. It is presented as an example for the following discussion.
I. The Administrative Core is a requirement of all Specialized Research Centers funded under a P50 mechanism. For this RFA, this core must include the following: 1) an Advisory Committee, 2) a Career Development Program, and 3) a Pilot Research Program. The consumer education component is not a requirement but is encouraged. NIH-funded Dietary Supplements Research Centers will be quickly identified as sources of information both for practitioners and consumers, as judged by the experience of the two Centers funded in FY 1999.
Why are detailed descriptions of pilot studies not allowed? The decision was based on the review of the applications submitted in response to the first RFA. P50s are very complex research projects. At a minimum, each proposal will include three R01-like research projects, an administrative core with at least three components, and a research resource core with at least two components. Many of the applications submitted last year also had very detailed descriptions of several pilot projects ("mini R01s"), further complicating the review process. A decision was made by Program Staff that it was more important for the applicants to describe the process used to propose, evaluate, choose, and implement pilot or feasibility projects. Applicants need to think about how the pilot program will help accomplish the research goals of their Center. Examples of pilot studies to be included in the Pilot Research Program are appropriate; avoid detailed descriptions. Applicants are, however, encouraged to present pilot data in their R01-like research projects.
What is the definition of a pilot or feasibility study? Would Phase I and Phase II studies be included? Since one of the R01-like research projects is likely to be a Phase I or Phase II study, it would be regarded as a primary research project rather than a feasibility study.
II. The grant proposal must also make provisions for a Research Resource Core. As stated in the RFA, this Core is defined as "shared research resources that enhance productivity or in other ways benefit investigators working to accomplish the common research goals of the Center". This implies that research goals for a proposed Center are clearly delineated in the grant application. A Center proposal is required to include two core components and one must be a botany/plant science core. A Dietary Supplement Research Center on Botanicals is expected to have expertise in botany/plant science.
Can pre-existing core facilities be used? Yes. Use of existing facilities and infrastructure is encouraged. Take maximum advantage of sharing existing facilities and space. If existing facilities/equipment are to be shared, a letter of commitment should be included in the proposal. Explain how access to shared resources will be determined.
III. The Research Project Components are individual R01-like research studies funded through the Center. The research projects are the heart of the Centers. Applicants should describe how the research projects would advance research and accomplish the goals of the Center. A Center application must include at least three but not more than four R01-like projects. Of the applications reviewed in response to the FY 1999 RFA, those with more than four research projects did not score well. The research projects do require preliminary data.
Can the individual research projects of the P50 application be simultaneously submitted to the CSR as investigator-initiated applications (i.e. R01s)? Yes, this is described in the RFA. If an R01 is submitted for two reviews, this should be disclosed under "other support" in the Center application. Be aware that the leader of a research project must relinquish the R01 and will not have the option to withdraw from the Center grant if both the Center application and the R01 application are found to be in the fundable range. If an investigator-initiated R01 is funded or an award is being made, that R01 cannot be included in the Center application.
Definition of Terms for the Grant Application:
As stated in the RFA, the Center Director is the only PI for the grant application. Co-Directors should not be designated in the grant application. The NIH staff will communicate only with the PI of the Center grant unless the PI (i.e. Center Director) gives permission to contact other investigators. R01-like research projects will be headed by Research Project Leaders. The RFA states that a Research Project Leader is equivalent to a PI on a standard NIH R01 grant application. This provision should allay concerns regarding the importance of official PI status in academic research. Research Cores will be headed by Core Leaders.
While a Center may represent a research consortium, a Center award will be made to one institution, the employer of the Center Director.
Important Dates:
Letter of Intent: March 25, 2000
Receipt Date: April 25, 2000
Review: late June or July
Council Review: August-September 2000
Award: September 2000
Will the RFA be reissued? If only one Center is funded, this RFA may be reissued. If additional Centers are funded, this may be the last issuance of an RFA to establish research Centers on botanicals.
Communicating With NIH Staff
Until April 25th applicants should contact Dr. Swanson, Dr. West, Ms. Suzanne White, or any of the IC contacts listed in the INQUIRIES section of the RFA. From the receipt date until the completion of the review by the SEP, applicants should communicate with the Scientific Review Administrator (SRA) responsible for organizing and conducting the review.
Submission of Application Materials
The grant application is due on the receipt date.
What happens if an applicant has important research findings relevant to the grant application, but the data were not available at the time of submission? The SRA may accept additional information from applicants, but this will be decided on a case-by-case basis. The first mailing to the reviewers is not likely to take place until a month after the receipt date. After the first mailing, additional materials will be mailed only to the primary and secondary reviewers. After the receipt date, the SRA will decide what materials will be included as part of the application. Reviewers have the right to request additional information from the applicant.
If I submitted an application for the RFA issued for FY 1999, is my application for this RFA (OD-00-004) considered a re-submission and do I have to respond to the previous review point by point? No. Since a SEP rather than a standing study section will review your application, it is considered as a new submission. However, some the reviewers from last year may be asked to review the applications submitted in response to the new RFA. Because the two RFAs are very similar, it would be useful to address the major criticisms of the previous applications.
THE REVIEW PROCESS
Triage
Initially the CSR and Program Staff will determine if grant applications are responsive to the RFA. If not responsive, the grant will not be reviewed. All applications that are responsive to the RFA will be reviewed and a written critique will be provided. However, some of these applications may not be discussed or scored by the SEP. If the number of applications responsive to the RFA far exceeds the number of awards likely to be made, it is NIH policy not to discuss and score every application. The effort of the SEP is best spent on applications likely to receive priority scores in a fundable range, usually defined as the best 50 percent of the applications received.
Review Criteria
Grant applications must meet all of the review criteria listed in the REVIEW CONSIDERATIONS section of the RFA. The SEP reviewers will focus on the R01-like research projects. Each research project will be given a priority score and each of the cores will be evaluated. Once the research projects have been discussed and scored, the reviewers will be asked to consider the Center as a whole. They will ask the following questions. Is the research described in the Center proposal likely to accomplish more than three or four separately funded R01s? Is the whole greater than the sum of the parts? This is described as synergy in the RFA. It includes a number of factors including the Center Directorís ability to administer a complex research program and provide scientific direction. Certainly, the Center should foster interdisciplinary research and the research scientists should be interacting. The final priority score could be the mean of the research project scores. It could be lower or higher, reflecting the reviewersí judgement as to the merit of the Center as a whole. In general, the reviewers are asked not to deviate by more than 0.5 units from the mean score of the research projects. For example, if the mean of the research projects is1.5 then the final priority score would be expected to range from 1.0 to 2.0.
Who will review the applications? The evaluation will be done by peer-review. The Program Staff are continually compiling a roster of potential reviewers. The Letters of Intent provide an early indication of the research areas that will require coverage. The first reading of the applications by the SRA and Program Staff will be more informative for purposes of identifying appropriate reviewers. An applicant may know of individuals with biases or other conflicts that might compromise the review of their proposal. Specific concerns should be expressed to the SRA before the review takes place.
Who will be excluded from the review panel? Any individual named on the budget page or listed as an investigator or collaborator will be considered in conflict and not eligible to serve on the review panel. Individuals who have published or worked with an applicant may serve on the review panel. However, if they were found to be in conflict on a particular proposal they would be asked to leave the room when that application is evaluated. The RFA specifically instructs applicants NOT to name any individuals on the Center Advisory Committee. Naming these individuals would reduce the number of potential reviewers, possibly to the point that the review would be compromised.
Why is there a limit to the number of research projects that can be proposed?
Based on the review of last year, applications with more than four research projects did not receive good scores and none were funded. Inclusion of a weak research project along with three or four strong proposals reflected poorly on the judgement of the PI.
How important is Institutional Commitment? Reviewers and the NIH recognize that strong institutional commitment is important to the success of such a complex research project. The award is a grant-in-aid and NIH expects a partnership. Applicants should be working to obtain and document institutional commitment.
What are some examples of Institutional Commitment? Laboratory space, matching funds for purchase of equipment, faculty appointments, co-funding of a regular seminar series. Institutional commitment also goes beyond financial considerations.
How is the Center Director factored into the review? The Center Director plays a critical role. The reviewers will ask the following questions. Does the PI have the capability to lead a group of scientists and manage such a complex project? Will the PI be able to devote adequate time to the Center? Is this person likely to move the research forward and achieve the goals of the Center? What is the track record of the PI?
Common Mistakes Made by Applicants
It is not appropriate for NIH staff to discuss the details of any specific application because of confidentiality concerns. However, Program Staff did observe a number of generic weaknesses in previous Center applications, and these are presented simply as examples of potential pitfalls in grant writing. Prospective applicants should also note that this list of examples does not preclude reviewers from finding other weakness in specific applications.
Do not dilute your efforts by proposing too many research projects or too many research resource core components.
Some applicants did not give adequate attention to statistical input. A statistician should be included in the design stage of the research projects. Do the research projects have adequate statistical power to provide answers to the research questions? As one approach, the applicant may choose to propose a biostatistical core that would be used in the design and analysis phases of most if not all research projects.
In the past, applicants did not score well if they did not represent an interdisciplinary effort or if the Center activities (particularly the research activities) were not integrated. The whole should be greater than the sum of the parts, indicating a high level of integration and interaction.
NIH requires that all clinical studies should include children and both genders unless there is a scientifically valid reason for exclusion. If a clinical study does not include children and both genders, the reason for the exclusion(s) must be provided. Detailed instructions about the requirements of the inclusion policy may be found in the instructions for PHS Form 398 and in the NIH/ORWH website.
Clinical Studies and INDS
Clinical studies are defined on the last page of the RFA. Phase III clinical studies are beyond the scope of this RFA. Phase I and II studies are encouraged. A controlled feeding study with an intermediate endpoint is considered a clinical study for the purpose of this RFA. Investigators are encouraged to conduct clinical studies that would lay the groundwork for Phase III trials.
The RFA states that "it is the sole responsibility of the applicant to obtain all necessary clearances from the Food and Drug Administration as required." In addition, applicants are strongly encouraged to consult their local Institutional Review boards (IRB) concerning IND status and the IRB approval process.
Are there any written guidelines regarding INDs for botanicals? No. The FDA is in the process of preparing a guidance document. The FDA is willing to advise applicants in developing IND programs for botanicals. Two individuals at the FDA have agreed to provide guidance to applicants for this RFA.
Robert J. Moore, Ph.D.
Senior Regulatory Scientist
Office of Nutritional Products, Labeling, and Supplements
Division of Compliance and Enforcement, HFS-456
200 C Street, SW
Washington DC 20204
Telephone: 202 205-4605
Fax: 202 260-8957
Email: [email protected]
Yuan-yuan Chiu, Ph.D.
Acting Director
Office of New Drug Chemistry
Center for Drug Evaluation and Research
Food and Drug Administration
Rm 13B-31, 5600 Fishers Lane
Rockville Md 20857
Telephone: 301 827-5918
Fax: 301 594-0746
Email: [email protected]
Institutional Review Boards (IRBs)
Applicants are strongly encouraged to begin preparations for local IRB review of any clinical study submitted as part of this RFA. Given cost constraints, it is likely that only one clinical study will be proposed as a research project. Applicants must submit documentation of IRB approval to arrive no later than 60 days after the application receipt date. A clinical study cannot be initiated without local IRB approval. If the IRB requires modifications to the clinical study protocol, these changes must be made and communicated to the SRA as soon as possible.
GRANTS MANAGEMENT ñ Suzanne White
Budget
Ms. Suzanne White, the grants management specialist, reviews the budget of each grant that will be scored by the SEP. The budget must accurately reflect how the money will be allocated. The written justification for the budget must match the numbers that appear on the budget page. The quality and accuracy of the submitted budget is a reflection of the management skills of the Center Director. All budget pages should be clearly identified by project name, core, or consortia identifier. A signed PHS398 face page should be included for each consortium (subcontract) activity. Facilities and Administration (F&A) Costs should be detailed on the checklist page for the parent grantee and for each consortium. If your institution does not have an F&A Costs (i.e., indirect costs) rate established with the NIH, please contact Ms. White as soon as possible.
The actual institutional base salary for personnel should be listed in the budget. The salary ceiling for NIH awards is $141,300 for FY 2000. The ceiling for FY 2001 has not been determined. Salaries are generally allowed to increase by 3% per year.
"Out year" (e.g., years 2-5) costs beyond 3% escalation must be clearly justified in writing. If the applicant does not justify these non-recurring costs, they will be eliminated from the budget. Changes in effort across the budget periods should be clearly described. Each core component (e.g., Administration Core) should have a budget page. All costs should be clearly justified in writing and supporting documentation may include separate budget pages for career development, pilot programs, and advisory committee costs.
Is it possible to obtain an example of a properly prepared budget?
An applicantís first resource for preparing a budget should be their grants and contracts office. Ms. White will also assist applicants with questions prior to submission of their grant application.
Can NIH provide guidance regarding budgets for pilot projects? The RFA states that no more than $100,000 (direct costs) per year may be devoted to pilot research projects. Applicant should determine the distribution of the funds among pilot projects.
Are subcontracts allowed? Yes. Since a grant award is made to only one institution, subcontracts are required if other institutions are involved. Indirect costs of subcontracts are considered direct costs of the primary institution. A signed PHS398 face page should be included for each consortium (subcontract) activity.
If a research project is done at another institution, is a subcontract required? Yes. Similarly, if a research resource core component resides at another institution, it will require a subcontract. Foreign institutions do not receive indirect costs. The applicant should justify collaborations with foreign institutions.
Can projected base salary increases be included in the budget? Yes.
Conflict of Interest
Each institution must have established procedures to identify, manage, reduce or eliminate any conflicting financial interest of an investigator involved for PHS funded research. This policy is detailed in the Code of Federal Regulation, 42 CFR Part 50, Subpart F and applies prior to the proposal being submitted, as well as post award. Applicants are cautioned that "other support" (present or pending research support), including private funding, must be fully disclosed.
INTELLECTUAL PROPERTY- Mr. Ted Roumel
The NIH has a Grant Policy Statement, which provides all of the terms and conditions of adhering to a grant award. The document can be found on the following web site
http://odoerdb2.od.nih.gov/gmac/nihgps/. Research findings must be made public, disseminated and shared with the research community.
The Office of Technology Transfer (OTT) provides guidance in the area of intellectual property. The OTT web site is http://ott.od.nih.gov/. NIH recently published a document on principles and guidelines for sharing unique biomedical research resources developed under NIH awards http://ott.od.nih.gov/RTguide_final.htm. Unique research resources (e.g., cell lines and knock out mice) are regarded as research tools and are intended to be shared.
Other Intellectual Property (Patents)
The Bayh-Dole Act of 1980 states that findings from federally funded research should be moved to commercially available channels. The intention was to stimulate economic development. An institution/organization must have some type of internal mechanism of recording inventions. Inventions must be reported within a certain time frame. A patent must be filed and the federal government must receive a royalty free license. The organization must acknowledge NIH funding on any patent. The organization must notify the NIH of any patent right decisions. If an organization does not pursue a patent, the federal government may do so. Failure of a grantee to comply with NIH requirements may result in loss of patent rights.
What is the reporting mechanism? Contact Dr. George Stone in the Office of Extramural Research. The report may be a hard copy or submitted electronically.
Regarding provisional statements, are invention disclosures included? Invention disclosures should be reported. When an institution believes that it has an invention, it should be reported.
Mr. Roumel is willing to serve as a contact person for issues related to intellectual property.
Mr.Ted Roumel
Assistant Director, OTT
Phone: 301 594-7700
Email: [email protected]
-------------------------------------------------------------------
NIH Staff
The Office of Dietary Supplements (301 435-2920)
Dr. Paul Coates-Director
Dr. Rebecca Costello-Deputy Director
Dr. Christine Swanson-Program Director for Dietary Supplements Research Centers
Dr. Mary Frances Picciano- Visiting Nutrition Scientist
Ms. Carol Haggans- Program Analyst
Mrs. Donna Allen-Office Manager
Ms. Leslie Johnson-Secretary
(Drs. Coates, Costello and Swanson attended the meeting.)
The National Center for Complementary and Alternative Medicine (NCCAM)
Dr. Neal West-Health Science Administrator/Program Officer
Dr. Eugene Hayunga-Health Scientist Administrator
The National Heart, Lung and Blood Institute (NHLBI)
Ms. Suzanne White-Grants Management Specialist
The Office of Technology Transfer (OTT)
Mr. Ted Roumel