Strategic Plan 2010–2014 Progress Report
Office of Dietary Supplements
National Institutes of Health
U.S. Department of Health and Human Services
January 2015
Table of Contents
- Comments from the Director, Office of Dietary Supplements
- Support for Research and Training
- Research and Training Portfolio
- Botanical Research Centers
- Mary Frances Picciano Dietary Supplement Research Practicum
- Programs Focused on Specific Nutrients and Nutrition/Supplement Interventions
- Vitamin D Initiative
- Population Studies of Folate and Vitamin B12 Status in the United States
- Iodine Initiative
- Evaluating Dietary Supplement Use in the United States
- Nutrition and Dietary Supplement Interventions for Inborn Errors of Metabolism
- Research Tools
- Analytical Methods and Reference Materials Program
- Dietary Supplement Databases
- Evidence-Based Review Program
- Collaborations with Other Federal Agencies
- Federal Working Group on Dietary Supplements
- ODS Collaborations with the U.S. Department of Agriculture (USDA)
- Department of Defense–ODS Nutrition Collaboration
- Dietary Reference Intake (DRI) Initiative
- Communications
- Communications Program
- References
- Appendix: Strategic Plan Goals Addressed by Ongoing and New ODS Programs
Comments from the Director, Office of Dietary Supplements
The Office of Dietary Supplements (ODS) is an active office within the National Institutes of Health (NIH). At nearly 20 years of age, it has been the focus of considerable interest from many stakeholders. ODS is committed to promoting good science to inform public health policy and to help consumers make informed decisions about their own health care. From time to time, ODS submits itself to public scrutiny, describes the outcomes of its investments, and offers an opportunity for the public to provide input into its plans. The current ODS Strategic Plan focuses on the following four goals:
- Goal 1: Foster research on the role of dietary supplements in health promotion and disease risk reduction. Examples include ODS sponsorship of workshops and evidence-based reviews on numerous dietary ingredients and leadership of government working groups. These activities promote the sharing of ideas and support efforts to advance dietary supplement research.
- Goal 2: Fund new research and training to expand the scientific knowledge base on dietary supplements. From fiscal year (FY) 2010 to FY 2014, ODS provided co-funding for 468 grants at a total cost of $67.3 million. For every dollar that ODS invested in research related to dietary supplements in FY 2013, other components of NIH invested $24. ODS also co-funds five Botanical Research Centers to conduct interdisciplinary research and provide training for graduate students and postdoctoral fellows.
- Goal 3: Support the development of tools for dietary supplement research. The ODS Analytical Methods and Reference Materials Program, which is devoted to this goal, brings together representatives from a broad range of government agencies, academic institutions, and the dietary supplement industry. Launched in June 2013, the Dietary Supplement Label Database is a research tool that provides information from the labels of dietary supplement products in the marketplace in a searchable format.
- Goal 4: Make up-to-date knowledge about dietary supplements publicly available. Here, the expansion of the ODS communications and outreach activities has paid off in measurable ways. ODS responds rapidly and effectively to consumer and media inquiries. ODS staff are regularly asked to comment on new and emerging research. Also, stakeholders regard the ODS communications vehicles—website, social media, fact sheets, videos, and other educational materials—as valuable resources.
As ODS considers revisions to its current strategic plan, two issues stand out:
- The ODS budget has been relatively stable over the last 10 years. To make the best use of that funding, setting priorities and critically evaluating the output and future of every ODS program are of paramount importance.
- ODS achieves its goals through collaboration. ODS therefore needs to continue building its communication and outreach activities with various stakeholder communities.
Although the ODS budget has remained essentially unchanged since 2004, its funding allows dietary supplements science to advance. For example, ODS has taken a leadership role in spearheading research initiatives that extend beyond NIH. These collaborations include activities with other agencies in the Department of Health and Human Services, such as the Food and Drug Administration, Agency for Healthcare Research and Quality, and Centers for Disease Control and Prevention. ODS also collaborates with other federal government agencies such as the Federal Trade Commission and U.S. Departments of Agriculture, Commerce, Defense, Justice, and Veterans Affairs. ODS partners with research communities in academia and industry. To ensure that scientific knowledge is effectively translated into public policy, such as public health or clinical practice recommendations, ODS provides funding and expertise to policy makers.
This report describes what ODS is currently doing to meet its legislative mandates, achieve its mission and the goals of its strategic plan, and enhance the health of the public. It provides details on each ODS program and activity between FY 2010 and FY 2014. The appendix provides a table that maps each ODS effort to its corresponding strategic plan goal and strategy. Lists of the publications and presentations by ODS staff members are available on the ODS website [1].
The comment submission period for developing the strategic plan for 2015–2020 is closed. However, ODS welcomes input from others, such as yourself, and invites you to visit our Contact Us page to submit a comment or question.
Paul M. Coates, Ph.D.
Director, Office of Dietary Supplements
National Institutes of Health
For further information contact: Anne L. Thurn, Ph.D., Office of Dietary Supplements, National Institutes of Health, 6100 Executive Boulevard, Room 3B01, Bethesda, MD 20892-7517, Phone: 301-435-2920, Fax: 301-480-1845, E-mail: [email protected].
A. Support for Research and Training
A1. Research and Training Portfolio
Contact: Cindy D. Davis, Ph.D., [email protected]
Introduction
Most of the National Institutes of Health (NIH) budget supports research grants. But unlike other NIH Institutes and Centers (ICs), ODS cannot issue grants directly. ODS therefore awards grants for research and training in collaboration with other NIH ICs by funding parts of these grants (co-funding). Approximately half the ODS budget supports research on dietary supplements.
ODS co-funding offsets some costs of research project budgets to allow investigators to undertake additional work that expands their project's scientific scope and impact. ODS also supports research through contracts, intra-agency agreements with other NIH units (including NIH intramural research programs, which are led by NIH staff researchers), and interagency agreements with other government agencies.
Research planning and training activities—such as workshops, conferences, and symposia—provide critical input on new directions for the ODS research portfolio. These events give researchers who receive ODS funding access to ODS staff expertise on dietary supplement matters and help ensure that the studies that ODS funds use well-characterized and high-quality products.
Activities
ODS-Funded Research Project and Training Grants in FY 2010–2014: Between FY 2010 and FY 2014, ODS co-funded 468 extramural research project grants (for researchers and organizations outside NIH) and training grants at a total cost of $67.3 million. Approximately half of this funding was for research on botanicals (herbs and other plant components), including studies conducted by the ODS Botanical Research Centers. The remaining research grants supported studies on vitamin D, iron, and other vitamins and minerals; fatty-acid supplements; other dietary supplements; and general nutrition (see pie chart below). Approximately 5% of the $67.3 million in total ODS extramural grant funding supported training programs.
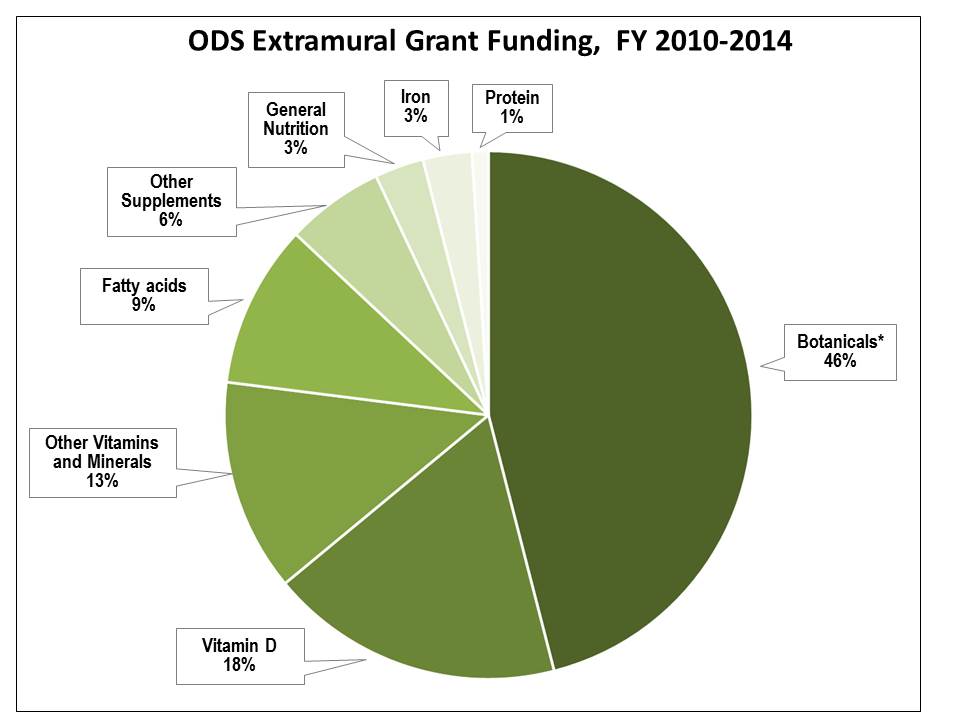
ODS co-funded 55% of these 468 grants with the National Center for Complementary and Alternative Medicine (NCCAM); National Heart, Lung, and Blood Institute; or Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). An additional 12 ICs were co-funding partners with ODS on the remaining 45% of research grants.
Examples of the topics of the research grants that ODS supported in FY 2010–2014 are as follows:
- Use of botanicals (including red ginseng, curcumin, and grape-seed extracts) to reduce the risk of different types of cancer
- Roles of fatty acids in obesity, heart disease, inflammation, psychiatric disorders, and cognitive function
- Causes and effects of iron deficiency (funded in partnership with the NICHD-sponsored iron and malaria initiative)
- Relationships among genes, certain nutrients, and various diseases
These examples give a sense of the types of research that ODS funds but do not represent the broad range of projects in the ODS grants portfolio. A list of all ODS-supported extramural grants, with links to funding details and abstracts, is available on the ODS website [2].
In 2011, an ODS-funded project on the contributions of dietary intakes of phosphatidylcholine, flora in the gut, and genetic susceptibility to the development of atherosclerotic heart disease received a Top 10 Clinical Research Achievement Award from the Clinical Research Forum [3]. Recent publications based on ODS-supported research have garnered worldwide media attention [4].
Funding for Interagency Agreements and Intramural Research: In FY 2010–2014, ODS funding for inter- and intra-agency agreements and intramural research projects totaled $31.4 million. This funding included support for contracts and other agreements with other federal agencies (including the U.S. Food and Drug Administration, Centers for Disease Control and Prevention, U.S. Department of Agriculture, and U.S. Department of Defense) and nongovernment entities (such as the Natural Medicines Comprehensive Database). These funds supported a broad range of activities, including the development, optimization, and validation of methods for analyzing dietary supplement ingredients; analyses of survey data on dietary supplement use; and development of dietary supplement standard reference materials. ODS also awarded funding to other NIH ICs to support intramural studies and the development and maintenance of nutrient databases.
Funding for Workshops and Conferences: Total ODS funding for workshops, symposia, conferences, and working group meetings in FY 2010–2014 was $528,000. These awards included sponsorship of international professional society meetings and support of meetings on the state of science in dietary supplements research.
Portfolio Analysis: ODS completed a portfolio analysis to better understand how its current funding portfolio relates to the entire NIH funding portfolio. To conduct this analysis, ODS staff compared funding by ODS to that by other ICs by types of dietary supplements studied and research category or condition. The results of this analysis, published in the Journal of Nutrition [5], helps ODS determine whether and how it is meeting stakeholder needs.
Formal Product Integrity Evaluations: In FY 2013, ODS began requiring formal product integrity evaluations to assess the product quality of all dietary supplements used in research before co-funding grants for that research. This requirement is based on the NCCAM Policy for Natural Products Integrity [6]. These evaluations ensure that investigators know the contents of the dietary supplements they are studying and are using the same product in all of their studies.
Publications List: ODS is compiling a list of publications that cite grants co-funded by ODS.
Identification of Co-Funding Opportunities: ODS cosponsors funding opportunity announcements related to dietary supplements with other NIH ICs. ODS grants-program staff search the NIH Guide for Grants and Contracts on a regular basis to identify funding opportunity announcements that are relevant to the ODS mission. ODS uses this information to identify funding opportunities to cosponsor with other ICs.
Seminars and Training for Intramural Scientists: ODS encourages its intramural awardees to attend the annual ODS Mary Frances Picciano Dietary Supplement Research Practicum. In addition, ODS expects these investigators to present their findings in a summer seminar for ODS staff. For example, four intramural awardees presented the results of their research at ODS staff meetings in the summer of 2012; another four did so in 2013 and three in 2014. In May 2014, ODS announced its Research Scholars Program [7]. This competitive program offers a 1-year opportunity for early-career intramural scientists to study the role of dietary supplements in health promotion and disease prevention. ODS will give primary consideration to proposals that stimulate dietary supplement research where it is lacking or lagging and to clarify gaps. ODS plans to issue 5 to 10 of these awards each year.
Revised Grants Management System: ODS deployed an updated version of its custom database application, Grants Efficiency Management System (GEMS), in June 2012. GEMS automates the processing, administration, and tracking of the office's funded research. The new version of GEMS offers faster performance and better query and reporting capabilities to help ODS program staff manage the office's grants and contracts.
A2. Botanical Research Centers
Contact: Barbara C. Sorkin, Ph.D., [email protected]
Introduction
Botanical dietary supplements [8] are made from plants valued for their health-related properties. Botanicals are sometimes called "herbal products" or "phytomedicines" (medicines derived from plants).
One in six Americans uses botanical dietary supplements. Yet much research is still needed to optimally identify and characterize these inherently complex and variable products and to understand their biological and clinical effects. The Botanical Research Centers (BRCs) program [9] promotes collaborative, interdisciplinary research on botanical dietary supplements with high potential for translation into practical benefits for human health.
Since 1999, the BRCs have conducted preliminary studies required for the design of definitive clinical trials on botanical dietary supplements. The BRCs have contributed to research to advance methods to characterize relevant botanical products, assess their biological activity, explore their mechanisms of action, and select botanical products to test in clinical trials. In addition, the BRCs have conducted preclinical and early-phase clinical research on botanicals, and they provide a rich environment for training and career development.
ODS selects the botanicals to study based on peer review of research proposals and frequency of use by the public and/or the potential of the research to contribute to public health either by providing data on safety or efficacy or by developing methods or approaches that may have broader applicability. The BRCs are advancing scientific knowledge about botanical products, including their safety, effectiveness, and mechanisms of action.
Activities
2010–2015 BRCs: In 2010, ODS, the National Center for Complementary and Integrative Medicine (NCCIM; formerly called the National Center for Complementary and Alternative Medicine [NCCAM]) [10], and the National Cancer Institute [11] issued 5-year awards for BRCs at Louisiana State University, University of Illinois at Chicago, University of Illinois at Urbana-Champaign (UIUC), University of Missouri, and Wake Forest University.
These BRCs are studying the safety and mechanisms of action of botanicals in women's health, metabolic syndrome, cancer, cardiovascular disease, and immune function, (including asthma and infectious disease). Between January 2010 and July 2013, investigators at these BRCs published 99 articles citing a BRC award in peer-reviewed journals.
Examples of the topics explored in BRC research include:
- The interactions between soy and the breast cancer drug tamoxifen
- The ability of certain green tea components to reduce the risk of Alzheimer's disease
- The development of new ways to analyze carotenoids
Pilot Projects: The BRCs fund pilot projects to help new investigators or experienced investigators who are new to botanical research generate enough data and publications to support competitive applications for independent research funding.
Collaboration with the ODS Analytical Methods and Reference Materials Program: To encourage the validation of novel, cutting-edge methods for analyzing botanical dietary supplements that have been developed through BRC research, ODS and NCCAM awarded supplementary funds to three BRCs between 2011 and 2012. The Journal of AOAC International published a report [12] on the first of these studies, which evaluated a method for measuring blood levels of digestion products from hops, a plant used in brewing beer. The other two awards focused on methods for determining the levels of compounds in plants that are believed to modulate metabolism or immune responses.
Support for Collaboration among BRCs: Activities to support collaboration among BRCs include annual in-person meetings for principal investigators and other researchers from all five BRCs. In 2010–2012, each of these meetings drew approximately 70–80 participants. The meetings have featured presentations and discussions about research progress, intellectual property rights that affect botanical research supported by the National Institutes of Health (NIH), and transparency and reproducibility of preclinical research. The meetings also include poster sessions.
In 2011, ODS and NCCAM provided additional funding through two small awards to support new research collaborations that apply expertise or capabilities at one BRC to the research questions under study at another. One of these awards supports new analyses of estrogenic components of wild yam samples using the plant collections and expertise of the Missouri Botanical Garden (a component of the University of Missouri BRC), the phytochemistry expertise of the National Center for Natural Products Research (a component of the UIUC BRC), the phytoestrogen expertise of the UIUC BRC, and the bioinformatics capabilities of the University of Missouri BRC.
In 2012, ODS launched a limited-access, password-protected, collaborative website for BRC personnel. The site provides contact information for all BRC personnel and a compilation of available BRC resources, including cutting-edge instruments, specialty software, archived human tissues, and specialized expertise.
Training: The training activities of the BRC program have included a travel award in 2012 to support attendance at the International Congress on Natural Products Research by a postdoctoral fellow at the University of Illinois at Chicago. Graduate students and postdoctoral fellows contribute to the research of the BRCs while being mentored by more senior researchers.
Since 2010, the BRCs have obtained several new NIH research training awards related to botanical dietary supplements research:
- The Louisiana State University BRC obtained a T32 training grant, Training in Botanical Approaches to Combat Metabolic Syndrome.
- The University of Illinois at Chicago BRC's principal investigator obtained a T32 training grant focused on natural product complementary and alternative medicine. This investigator also obtained funding to support an underrepresented minority postdoctoral fellow. Both of these awards are supported by NCCAM with co-funding from ODS.
- The UIUC BRC investigators obtained funding to provide enriched training for postdoctoral fellows through an existing environmental toxicology training grant from the National Institute of Environmental Health Sciences.
- The Wake Forest University BRC obtained additional funding to support the training of a new, underrepresented minority graduate student. Also, one of the lead investigators of this BRC was awarded renewed funding for a T32 training grant focused on lipid metabolism, inflammation, and chronic diseases.
Communications and Outreach: To communicate with the research community and the public about its activities, each BRC maintains a website. Several BRCs also offer training programs for the scientific community and the general public. For example, the University of Illinois at Chicago BRC offers annual public tours of the university's Dorothy Bradley Atkins Medicinal Plant Garden. Each annual tour features a presentation by a renowned speaker on botanical dietary supplements. In 2012, this BRC co-hosted the 8th Annual National Health Research Institute (NHRI) Scientific Symposium on the Effectiveness of Natural Products for Women's Health with the NHRI and the American Nutrition Association. The Louisiana State University BRC offers annual public education programs on botanical products and health.
Botanical Research Expert Panel: The BRC Program had a decennial, formal, external review in 2003. To obtain expert input on how to most effectively focus this program, ODS and NCCAM assembled a panel of nine experts in botanical or biomedical research or in the medical or traditional practice of phytotherapy (health promotion or treatment with plant products). The panel met in person on April 29, 2013, after a series of preliminary discussions. During this meeting, attended by representatives of six NIH Institutes as well as staff from other federal agencies, the panel addressed questions ranging from the most important challenges and opportunities currently facing the botanical research field to issues to consider in prioritizing botanical research over the next few years.
ODS and NCCAM published an executive summary [13] of the expert panel's discussions in July 2013. The first action following the expert panel discussions, with approval by NCCAM's Advisory Council, was the publication of a concept for a new funding opportunity announcement for BRCs [14]. New awards are expected in 2015.
Future Activities
If NIH implements the plan described in the concept, future BRCs will leverage their unique ability to elucidate the mechanisms of action of complex botanical products, the chemical entities that contribute to these mechanisms of action, and the interactions among those chemical entities as they interact with human biology. To achieve these objectives, the BRCs will use state-of-the-art technologies and approaches, develop new methods, and adapt cutting-edge technologies from allied fields.
A3. Mary Frances Picciano Dietary Supplement Research Practicum
Contact: Regan L. Bailey, M.P.H., Ph.D., R.D., [email protected]
Introduction
The annual Mary Frances Picciano Dietary Supplement Research Practicum is the major ODS educational initiative. The practicum most visibly demonstrates ODS's desire to create opportunities for dietary supplement and nutrition research training and career development by bringing together approximately 100 attendees to the main campus of the National Institutes of Health (NIH) each year.
Activities
The annual practicum provides a thorough grounding in issues, concepts, unknowns, and controversies pertaining to dietary supplements and supplement ingredients. First held in May 2007, the practicum consists of a series of presentations organized by topic with extensive time for questions and discussion. Topics addressed include:
- Supplement use in the United States and reasons for use
- Regulatory framework governing supplement development and marketing
- Case studies of various nutrients and bioactive ingredients commonly used in the United States
- Key concepts for conducting and evaluating scientific research on dietary supplements
- Supplement quality
- Health effects of foods and supplements
- Policies and advice about supplement use based on science
Each year, ODS reviews and modifies the practicum agenda to change its subject matter and focus, respond to attendee evaluations, and ensure speaker availability. Between 2007 and 2011, the practicum took place over 4.5 days (Monday through Friday). Starting in 2011, ODS updated, consolidated, and compressed the practicum into a 3.5-day program.
Eligibility for the practicum was originally limited to full-time academic faculty members, doctoral students, postdoctoral researchers, and fellows in health-related disciplines as well as selected government employees and contractors. In 2012, ODS expanded eligibility for the practicum to health-care providers and scientists with a postgraduate degree whose work involves dietary supplements; master's degree students; and medical, dental, and nursing students. Full-time faculty and students at the doctoral level and beyond receive priority admission; others are admitted as space permits. Each year, the number of applicants has exceeded the space available.
From 2010 through 2014, 463 participants have attended the practicum. Of these, 145 were academic faculty members; 95 were health care practitioners; 181 were doctoral students, postdoctoral researchers, or fellows; and 14 had other backgrounds.
Most ODS senior scientific staff members speak at the practicum and moderate sessions. The practicum faculty also consists of experts from NIH; academic institutions; and federal regulatory agencies, such as the U.S. Food and Drug Administration. The sessions include a panel with representatives from the dietary supplement industry, consumer groups, and a journalist who covers dietary supplements.
Evaluation
The practicum is evaluated annually by the participants. In 2014, as in previous years, participants rated 100% of the faculty as excellent or very good. In 2012, ODS commissioned a survey that was sent to every practicum attendee from 2007 to 2011; the survey had a 40% response rate. Approximately 75% of the respondents reported that they were initially interested in participating in the practicum for research purposes. About half attended to enhance their ability to teach about dietary supplements and for networking opportunities. On a scale of 1 to 4 (not very to very valuable), the average rating of the perceived value of the practicum at the time of attendance was 3.54. The average rating of the practicum's perceived value when they completed the survey was 3.44, suggesting that the knowledge they gained had an enduring impact. Approximately 69% of respondents stated that they used the knowledge gained in the practicum to create new course materials and enhance their own teaching or research on the topic. Many agreed that the practicum had expanded their knowledge of the dietary supplement industry, dietary supplements in general, and the regulation of these products.
Future Activities
As a result of budget constraints, ODS has had to make some difficult decisions in prioritizing our spending in 2015. Because of this, ODS is canceling the Dietary Supplement Research Practicum in 2015. We will re-evaluate this decision in 2016.
B. Programs Focused on Specific Nutrients and Nutrition/Supplement Interventions
B1. Vitamin D Initiative
Contacts:
Christopher T. Sempos, Ph.D., [email protected]
Christine Taylor, Ph.D., [email protected]
Introduction
ODS leads and sponsors several efforts to advance scientific understanding of the importance of vitamin D to health. In 2010–2014, these activities included:
- Reviewing the health effects of vitamin D
- Measuring vitamin D exposure and status in the U.S. population (with the Centers for Disease Control and Prevention [CDC])
- Developing research methods
- Evaluating biomarkers
- Funding a broad range of research
- Developing standardized measurement procedures and reference materials
- Establishing the international Vitamin D Standardization Program (VDSP) with CDC and the National Institute of Standards and Technology (NIST)
- Planned a conference on evidence-based decision making related to vitamin D in the context of primary care
Activities
Review of the Health Effects of Vitamin D: ODS was the primary funder of an evidence-based review of the health effects of vitamin D and calcium by the Agency for Healthcare Research and Quality (AHRQ). This review [15], published in 2009, informed the deliberations of an Institute of Medicine (IOM) panel of scientists that evaluated the dietary reference intakes (DRIs) for these two nutrients. ODS contributed intellectually and financially to the IOM panel, as did other agencies of the U.S. and Canadian governments.
Issues highlighted by the DRI report [16], which the IOM released in 2011, included the need for surrogate outcomes, reliable indicators of exposure, and standardization of the measurement of 25-hydroxyvitamin D [25(OH)D], a marker of vitamin D status and exposure. ODS has used the research agenda from the DRI report to plan its activities related to vitamin D.
In 2013, ODS contracted again with AHRQ to update the 2009 review with evidence from studies published through 2013 that were related to outcomes associated with vitamin D alone or in combination with calcium. In the updated review [17], issued in September 2014, the authors concluded that despite the availability of new research, data remained inconclusive regarding the health benefits of vitamin D other than those related to bone. The update will be useful for future IOM activities, and it served as a foundation of the ODS-sponsored conference, Vitamin D: Moving Toward Evidence-Based Decision Making in Primary Care (see below under "future activities") held in December 2014.
Measurement of Vitamin D Exposure and Status in the U.S. Population: ODS has supported and collaborated with Centers for Disease Control and Prevention (CDC) to measure serum levels of 25(OH)D in the National Health and Nutrition Examination Survey (NHANES) since 2000. This work included sponsoring a roundtable meeting to evaluate the extent of drift (or discrepancies between laboratory results of vitamin D measurements in serum samples over time) and ways of eliminating this drift. In addition, ODS and CDC developed a long-term plan to change the laboratory method used in NHANES to measure 25(OH)D levels as well as a study design for calibrating the old values to the new ones [18].
ODS staff conducted research to evaluate current levels of vitamin D nutriture and trends over time [19-22]. In addition, ODS evaluated the appropriateness of using the probability approach (a statistical method) with 25(OH)D to assess the proportion of the population with inadequate vitamin D intakes [23].
Methods Development: ODS staff conducted research to develop methods for calculating total vitamin D intake from the diet and from supplements and evaluate the impact of within-person error on estimates of the prevalence of vitamin D insufficiency. ODS staff also evaluated the prevalence of vitamin D deficiency, insufficiency, sufficiency, and possible overload using NHANES 25(OH)D data and the DRI committee's definitions of these terms [24]. In addition, ODS and the U.S. Department of Agriculture explored the potential role of 25(OH)D in foods with respect to vitamin D status and outlined methodologies needed to clarify its role [25]. ODS is pursuing research to elucidate potential outcomes under varying fortification scenarios for vitamin D as well as the appropriateness of methods using changes in the distribution of status measures before and after fortification for one nutrient (folate) as a predictor for changes before and after fortification for another nutrient (vitamin D). ODS has submitted a manuscript summarizing these findings to a peer-reviewed journal.
Biomarkers Evaluation: ODS facilitated and conducted research on the validity and utility of biomarkers of vitamin D status as surrogate and clinical endpoints. This research included assessments of the association between serum 25(OH)D levels and hyperparathyroidism, a condition that can cause osteoporosis and other health problems, and death from all causes. This work has resulted in several published papers [26-28] and presentations at scientific meetings.
Extramural Research: In 2010–2014, ODS co-funded 85 grants for basic, epidemiological, and clinical research on vitamin D. These studies addressed such issues as vitamin D metabolism and the relationship between vitamin D status markers and biological and clinical outcomes, including cancer; cardiovascular diseases; chronic kidney disease; diabetes; and endocrinologic, immunologic, ophthalmologic , and metabolic bone diseases. Extramural funding for vitamin D research in 2010–2014 by ODS totaled $11.9 million.
Development of Standardized Vitamin D Measures and Reference Materials: ODS collaborated with NIST to develop a reference measurement system for vitamin D that included a reference measurement procedure and Standard Reference Materials® (SRMs). Reference measurement procedures and SRMs are used to make sure that measurement procedures yield consistent and accurate results. ODS also supported the development of a study design for standardizing past vitamin D measurements [29].
Currently, ODS is working with NIST to:
- Support development by NIST of vitamin D SRMs [SRM 972, vitamin D in serum; SRM 972a, replacement for SRM 972; and SRM 2972, 25(OH)D2 and 25(OH)D3 calibration solutions in ethanol] that laboratories use to calibrate their vitamin D measurement procedures
- Develop a new low-cost, high-throughput reference measurement procedure that laboratories can use to measure the amounts of different chemical forms of vitamin D [25(OH)D2, 25(OH)D3, 3-epi-25(OH)D2, 3-epi-25(OH)D3, and 24R,25(OH)2D] in serum
- Determine normal levels of the 3-epimer of 25(OH)D2 in serum and support research to evaluate the biological significance of this chemical form
- Conduct the NIST-National Institutes of Health (NIH) Vitamin D Metabolites Quality Assurance Program, which compares the results of vitamin D measures in human serum samples from different laboratories that use different methods
- Convert the Vitamin D External Quality Assurance Scheme (DEQAS) to a "trueness" quality assurance program that permits laboratories to compare their results with the true concentration of vitamin D in blood samples. Through an ODS-led effort, NIST is assigning vitamin D levels to DEQAS blood samples using NIST's reference measurement procedure
- Design a study to determine whether the vitamin D levels measured by research laboratories in pooled blood (e.g., SRMs and DEQAS materials) are the same as in blood samples from individual patients
- Develop a reference measurement system to standardize the measurement of 25(OH)D from plant- and animal-based foods
Establishment of the International VDSP: In 2010, ODS, CDC, and NIST jointly established the international VDSP to standardize the laboratory measurement of vitamin D status in national health surveys worldwide. This effort was the result of a meeting sponsored by ODS in 2010. ODS subsequently sponsored a meeting of the VDSP's protocol development subcommittee in 2011 and a VDSP meeting in 2012 [30]. The results from the participating national health surveys will help promote standardization of vitamin D measurements by demonstrating the beneficial effects of standardization [29, 30]. ODS coordinates this program.
ODS, in collaboration with CDC and NIST, designed and conducted a VDSP study that compared the vitamin D measurement results of different laboratories. Together, they also designed and conducted a VDSP commutability study to determine whether the vitamin D levels measured by research laboratories in pooled blood from many patients are the same as the results in blood samples from individual patients. The results of both studies will be published in peer-reviewed journals in 2015.
With ODS support, CDC established a program in 2012 to certify laboratories that measure vitamin D levels in blood, and this program will complement ongoing VDSP efforts. Through this program, efforts are underway to ensure that manufacturers' assays for measuring vitamin D levels in blood yield the same results as the NIST reference measurement procedure. This effort is essential for VDSP because research laboratories have little control over the kits produced by manufacturers that laboratories use to measure vitamin D levels. Moreover, the NIST-NIH Vitamin D Metabolites Quality Assurance Program and DEQAS help clinical and research laboratories use the vitamin D kits properly. These two arms of VDSP are promoting laboratory standardization of 25(OH)D measurement worldwide.
In 2013, VDSP began comparing the results from the national health and nutrition surveys of countries participating in the VDSP for vitamin D levels in blood and reported vitamin D intakes.
Conference—Vitamin D: Moving Toward Evidence-Based Decision Making in Primary Care: ODS cosponsored a conference with other federal partners on vitamin D and evidence-based decision making in primary care, which took place on December 2–3, 2014 [31]. The conference addressed screening for vitamin D insufficiency, interpreting laboratory measures, and determining interventions (such as supplementation) using evidence-based approaches to decision making in primary care practice. An executive summary of the conference will provide an overview of the discussions, next steps, and research gaps.
Future Activities
ODS will participate in a European Union initiative to study the measurement of vitamin D in blood and vitamin D intake data in participating nations to inform the development of vitamin D intake recommendations in Europe. ODS will lead a vitamin D standardization activity that will address standardization of vitamin D measurement results.
In 2014, planning began for the second larger and more extensive VDSP study of commutability in a larger sample of vitamin D assays from more commercial manufacturers than before. This 1-year study is scheduled to begin in spring 2015.
B2. Population Studies of Folate and Vitamin B12 Status in the United States
Contacts:
Regan L. Bailey, M.H.P., Ph.D., R.D., [email protected]
Elizabeth Yetley M.P.H., [email protected]
Introduction
"Folate" is an umbrella term used to refer to the different forms of this B vitamin. Food folate is the form that naturally occurs in food sources. Folic acid is the form found in fortified foods and dietary supplements. "ietary folate" refers to both food folate and folic acid in fortified foods, whereas "total folate" includes all dietary and supplemental exposure to folate and folic acid.
Folic acid supplementation before conception and during early pregnancy reduces the risk of neural tube defects; these birth defects affect the brain, spine, or spinal cord [32, 33]. For this reason, the governments of both the United States and Canada established national folic acid fortification programs to increase the intake of this nutrient in females of reproductive age [34-36]. Rates of neural tube defects decreased in both the United States [37] and Canada [38-40] after these programs began, but these fortification programs have also increased the folic acid intakes of people who are not females of reproductive age. Although higher intakes of folate from foods might prevent some cancers and cardiovascular disease, they could increase the risk of colorectal cancers [41, 42] and mental impairment [43, 44]. Therefore, continual monitoring of total folate intake from food and dietary supplements is a priority for ODS.
Vitamin B12 deficiency can cause irreversible brain and spinal cord damage. Its symptoms—such as forgetfulness, changes in gait, depression/mood alterations, and fatigue—are often mistakenly attributed to aging. Given the aging of the U.S. population, monitoring of vitamin B12 deficiency is the focus of several ODS activities.
Activities
Roundtable Meeting: In July 2010, ODS convened a roundtable in collaboration with the CDC of 23 experts in folate and vitamin B12 assessment, clinical laboratory science, and biostatistics and 10 scientists from U.S. government agencies. The roundtable discussed the measurement of folate and vitamin B12 status biomarkers in NHANES and identified research gaps in the field. The group published nine articles based on the meeting in a supplement [18] to the American Journal of Clinical Nutrition.
Standard Reference Material® (SRM) for Serum: ODS and National Institute of Standards and Technology (NIST) jointly developed an SRM for measuring levels of the amino acid homocysteine and folate in frozen human serum. Homocysteine levels are higher in people with folate deficiency, and high homocysteine levels are associated with an increased risk of cardiovascular disease and dementia. Laboratories can use this SRM to ensure that their measurements of homocysteine and folate in serum are accurate.
Fact Sheet for Health Professionals: ODS issued a new fact sheet on folate for health professionals in 2012 [51] and English and Spanish consumer versions of this fact sheet in 2013 [52, 53].
Ongoing Activities
Analyses of National Health and Nutrition Examination Survey (NHANES) Data on Folate and Vitamin B12 Intakes and Status: ODS scientists collaborate with experts at the Centers for Disease Control and Prevention (CDC) to review and analyze NHANES data on folate and vitamin B12 intakes and status. They make the results of these analyses available to researchers through publications in peer-reviewed journals and scientific presentations at national and international meetings.
Studies of NHANES data that ODS completed during the current strategic planning period include:
- Comparison of folate levels in 1988–1994, before folic acid fortification became mandatory, and in 1999–2010, when the folate fortification program was in place [45]
- Identification of the most appropriate methods to characterize low vitamin B12 concentrations in U.S. adults [46, 47]
- Examination of the relationship between serum levels of unmetabolized folic acid and folic acid intake from foods and supplements [48-50]
Future Activities
ODS will continue to analyze NHANES data on folate and vitamin B12 status in the United States and address related measurement issues. ODS and its CDC colleagues plan to publish the results of recently completed studies on the factors associated with folate levels that are linked to a lower risk of neural tube defects, the relevance of unmetabolized folic acid to human health, and the effects of low vitamin B12 status on bone.
B3. Iodine Initiative
Contact: Abby Ershow, Sc.D., R.D., [email protected]
Introduction
Iodine is an essential nutrient and a component of thyroid hormone. The iodine status (i.e., adequacy or deficiency) of populations and individuals varies with local geographic features, availability in the food supply, and use of fortified foods and dietary supplements. Although iodine deficiency is rare in the United States and Canada, it can have serious effects. For example, iodine deficiency can cause lower-than-average IQ in infants and children, decreased ability to work and think clearly in adults, and during pregnancy, birth defects in newborns.
ODS developed its Iodine Initiative in response to the concern that many pregnant women were not using supplements containing iodine. As a result, these women were at risk of inadequate intake at a time of high physiologic demand.
ODS activities in iodine nutrition focus on supporting research, methodology development, and research-related resources that can provide a scientific base for understanding how best to improve iodine status in individuals with low to moderate risk of deficiency.
Activities
Expert Panel Meetings:
- 2011 Workshop for Iodine Experts and Federal Representatives: ODS sponsored this workshop, which brought together iodine experts and representatives from the National Institutes of Health (NIH), other agencies of the Department of Health and Human Services, and Health Canada, to begin developing an NIH iodine research initiative. Participants identified research needed to inform the development of new dietary reference intakes for iodine. Workshop leaders published a summary in the Journal of Nutrition [54].
2013 Symposium at Experimental Biology: ODS staff led a symposium during the scientific sessions hosted by the American Society for Nutrition's annual meeting at Experimental Biology 2013. This symposium summarized the results of recent clinical trials that have evaluated the efficacy of iodine supplementation in populations at greatest risk of iodine deficiency. The symposium leaders published a summary in Advances in Nutrition [55].
2014 Meetings: ODS held three Roundtable meetings in 2014. ODS scientists will submit a summary of the presentations and discussions to a journal for publication in 2015. ODS is also preparing executive summaries of the three meetings that will be posted on the ODS website in early 2015.
-
- Roundtable 1—April 22–23, 2014: The first workshop focused on the assessment of iodine intake from both foods and dietary supplements.
- Roundtable 2—July 22–23, 2014: The second workshop addressed laboratory assessments of iodine status, including assessments related to thyroid function, particularly in relation to the needs of high-risk groups (pregnant and lactating women, infants, and young children).
- Roundtable 3—September 22–23, 2014: The third workshop addressed iodine status concerns in pregnant women, the potential impact of suboptimal status on pregnancy outcomes and child development, and suitable study designs for evaluating and improving iodine status in regions with differing levels of risk and among various high-risk groups (infants, adolescent girls, women of reproductive age, lactating women, and older adults).
Characterization of Population-Level Iodine Intake: The Centers for Disease Control and Prevention (CDC) monitors the iodine status of the U.S. population through the National Health and Nutrition Examination Survey (NHANES). The CDC evaluates iodine status in NHANES by examining the distribution of urinary iodine concentrations of spot urine collections [56]. ODS analyzed NHANES data to determine the proportion of pregnant women advised by their physicians to take supplements containing iodine (80%). Only 20% of these women used iodine-containing supplements, reinforcing concerns about whether iodine intake is adequate in this population [57].
Research Resource and Methodology Development: The ODS Analytical Methods and Reference Materials Program [58] collaborates with other government groups, including the U.S. National Institute of Standards and Technology and the CDC. The program works to improve existing analytical methods and develop new approaches to facilitate the evaluation of iodine status.
Informational Materials for Consumers and Health Professionals: In 2011, ODS developed iodine fact sheets for health professionals [59] and, in both English and Spanish, for consumers [60, 61].
Biomarkers of Nutrition for Development (BOND) Program: ODS has supported the BOND program, led by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of NIH, to harmonize the processes for choosing biomarkers to use for research, program development and evaluation, and evidence-based policies [62]. The Iodine Expert Panel was one of the first programs that the BOND program developed, and ODS staff participated in the panel's 2011 meeting [63]. The iodine panel report, published in August 2014, summarizes the state of the science and identifies critical research priorities [64].
Future Activities
Good methods for assessing iodine levels, better understanding how to measure iodine status in populations and individuals, and increasing confidence in cut points for iodine sufficiency and insufficiency are needed. Additional efforts by ODS and other stakeholders focused on intake, assessment, status, and ultimately, design of appropriate intervention studies will inform the research agenda and future policy making.
B4. Evaluating Dietary Supplement Use in the United States
Contact: Regan L. Bailey, M.P.H., Ph.D., R.D., [email protected]
Introduction
Dietary supplement use is widespread in the United States. The two major surveys of dietary supplement use and reasons for use are conducted by the Centers for Disease Control and Prevention (CDC):
- The National Health and Nutrition Examination Survey (NHANES): Continuously surveys the health status and health-related behaviors of the U.S. civilian population
- The National Health Information Survey: Another nationally representative, periodic survey of the U.S. civilian population that asks about the use of complementary and alternative medicine modalities
Sources of data on health status and mortality include NHANES and the National Death Index.
Several ODS scientists, with collaborators on a study-by-study basis, gather information from the datasets and subject it to various statistical and other interpretive analyses to answer questions of interest pertaining to dietary supplement use in the United States. Collaborators in this research include scientists at the National Institutes of Health (e.g., from the National Cancer Institute and Office of Disease Prevention), other government agencies (e.g., CDC and U.S. Department of Agriculture), academia, and private consulting companies.
Activities
ODS published the results of the following analyses of data on nutrient intakes from food and dietary supplements in the U.S. population between 2010 and 2014:
- Total folate and folic acid intakes from foods and supplements between 2003 and 2006 among adolescents and adults [50]
- Total usual calcium and vitamin D intakes from foods and supplements among children and adults between 2003 and 2006 [24]
- Dietary supplement use between 2003 and 2006 among adults and children [21]
- Relative contributions of micronutrients from food, fortified and enriched food products, and dietary supplements among children and adults between 2003 and 2006 [65]
- Association between dietary supplement use and intakes of minerals from food sources among adults between 2003 and 2006 [66]
- Vitamin intakes from foods by dietary supplement use and contributions of supplements to ability to meet or exceed recommended intake levels among adults in 2003-2006 [67]
- Effects of dietary supplements on micronutrient sufficiency in children and adolescents between 2003 and 2006 [68]
- Dietary supplement use among pregnant women between 1999 and 2006, with a focus on iron and folic acid use and assessment of red-blood-cell folate status [69]
- Dietary supplement use among children in 2007 and predictors of supplement use [70]
- Prevalence of use of iodine-containing dietary supplements between 1999 and 2006 among reproductive-age women [57]
- Effects of update to the daily values on food labels on future food fortification and resulting nutrient intakes in the U.S. population [71]
- Contributions of fortified foods to nutrient intakes in the diets of children and adolescents between 2003 and 2006 [72]
More than half of adults use dietary supplements, although the reasons for this use had not been previously examined in U.S. adults using nationally representative data. One ODS study examined why adults take dietary supplements [73], and another examined why children take these products [74]. The most common reasons for both populations are to improve or maintain health. An important finding from both studies was that most supplement use is not based on recommendations from health-care providers.
The focus of current work in this area is to examine the possibility that relationships exist between dietary supplement use and health status, disease prevention, and mortality. ODS has a paper in press in the Journal of Nutrition on the use of multivitamin (MV) and multivitamin/multimineral (MVM) dietary supplements among adults between 1988 and 1994 and mortality from cardiovascular disease through 2011. The study found a strong and consistent relationship between the use of MVM supplements and a reduced risk of cardiovascular disease mortality in U.S. adults, especially women. A related current study focuses on MV and MVM use and mortality due to cancer.
Several of the publications described above address the contributions of fortified foods to total nutrient intakes. ODS scientists and colleagues have studied the implications, opportunities, and challenges of food fortification for nutrient adequacy and health. During this strategic planning period, ODS researchers have submitted three papers to a journal and published one on this topic [70].
In August 2013, ODS co-sponsored a workshop, The Use and Biology of Energy Drinks: Current Knowledge and Critical Gaps, to address the growing consumption of energy drinks and evaluate their claimed effects on alertness, fatigue, cognitive function, physical energy, and weight loss or maintenance. The workshop included an analysis of the caffeine content in energy drinks and dietary supplements presented by ODS scientist Dr. Regan Bailey [75]. Dr. Bailey also spoke about the caffeine content of dietary supplements and foods at an August 2013 workshop on caffeine sponsored by the Institute of Medicine [76].
Future Activities
ODS scientists will continue to investigate possible relationships between dietary supplement use, disease risk, and mortality. ODS will consider redoing its analysis of 2003–2006 NHANES data using updated data from NHANES for 2007–2010 to determine whether the earlier published findings are still valid or learn how they may have changed.
B5. Nutrition and Dietary Supplement Interventions for Inborn Errors of Metabolism
Contact: Kathy Camp, M.S., R.D., C.S.P., [email protected]
Introduction
Inborn errors of metabolism (IEM) are rare disorders caused by genetic mutations. These mutations lead to the absence or abnormal production of enzymes involved in processing protein, carbohydrate, or fat. Left untreated, these disorders can lead to brain damage, poor growth, impaired physical and intellectual development, or even death. Each IEM that can be managed by nutritional interventions requires lifelong dietary management through specialized medical foods, dietary supplements, and limited intake or avoidance of certain foods.
The scientific evidence supporting many of these dietary strategies is limited. ODS established the Nutrition and Dietary Supplement Interventions for IEM (NDSI-IEM) initiative in 2010 in collaboration with the Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The purpose of the NDSI-IEM is to identify gaps in research on the safety, efficacy, and effectiveness of nutritional treatments, including dietary supplements, for IEM. With input from a broad range of interested and involved parties, this highly collaborative program is developing strategies for identifying research needed to inform clinical practice guidelines that benefit persons with IEM.
Activities
NDSI-IEM Federal Partners Meeting: In January 2011, NIH convened a meeting of federal partners to engage the metabolic disease research community and develop a plan to promote evidence-based research on nutrition and dietary supplement interventions used in IEM. The federal partners included representatives of several NIH Institutes and Centers (National Heart, Lung, and Blood Institute; National Human Genome Research Institute; National Institute of General Medical Sciences; National Institute of Neurological Disorders and Stroke; National Institute on Deafness and Other Communication Disorders; Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD]; and ORDR), Food and Drug Administration (FDA), and Health Resources and Services Administration (HRSA).
Workshop for IEM Stakeholders: ODS sponsored a workshop in December 2011 for content experts, advocacy groups, industry, and research funders and regulators to address challenges and share successes in IEM research. This meeting featured presentations and discussions of current IEM research, FDA regulations governing products to treat IEM, and research gaps.
Follow-up activities to this meeting have included:
- Creation of a core planning group to prioritize the workshop recommendations for short-term and long-term activities
- Updates to the NDSI-IEM Web page on the ODS website
- Publication of an article, based in part on the workshop, about medical foods for IEM in Molecular Genetics and Metabolism [77]
- Catalog of the IEM and their treatments that are most important to the metabolic disease research community to understand the extent of nutrition and dietary supplement use in these disorders
Portfolio Analysis: In addition to the follow-up activities to the stakeholder workshop described above and in response to recommendations from that workshop, ODS recently reviewed the NIH research portfolio to identify IEM-related grants and other awards.
State-of-the-Science Conference on Phenylketonuria (PKU): NICHD, ODS, and ORDR sponsored this conference in February 2012. Speakers presented published data and clinical findings to inform future research needs and the development of clinical practice guidelines by professional organizations. Conference proceedings were published in 2014 [78].
Meeting of PKU Guideline Developers: In August 2012, ODS hosted a meeting of representatives from genetic and metabolic professional organizations who are developing clinical practice guidelines. After hearing presentations from NICHD and the Agency for Healthcare Research and Quality, participants collaborated to develop complementary nutritional and medical guidelines for PKU. The process for developing the guidelines was presented at the Annual Clinical Genetics Meeting of the American College of Medical Genetics in March 2013. The guidelines were published in 2014 [79, 80].
Improving Patient Access to Medical Foods: The NDSI-IEM and NICHD formed a medical foods working group in collaboration with HRSA and the Assistant Secretary for Policy and Evaluation of the Department of Health and Human Services. The group evaluated and synthesized existing data on medical foods and identified research needed to improve patient access to medical foods.
Nutritional Interventions in Primary Mitochondrial Disorders: Developing the Evidence Base: In collaboration with NICHD and ORDR, ODS held a workshop in December 2014. The workshop's goals were to explore the use of nutritional interventions, including dietary supplements, in mitochondrial disorders; identify gaps in knowledge; develop a research agenda; and identify research opportunities that will promote an evidence base for use of these therapies in primary mitochondrial disorders. A summary of the meeting will be posted on the ODS website in early 2015.
Other Collaborative Activities: NDSI-IEM staff have been working with FDA and other NIH Institutes and Centers to:
- Identify novel study designs that will make research on these rare disorders possible
- Determine how to leverage or modify the current regulatory infrastructure to increase the success rate of IEM-related grant applications and the development of new treatment products
- Identify alternative approaches to assess clinically meaningful outcomes or endpoints for IEM in areas in which valid biomarkers do not exist
- Catalog resources at NIH, FDA, and elsewhere to support IEM research
ODS with FDA, other NIH Institutes and Centers, and metabolic disease clinicians and researchers have published an article discussing some of these issues [81].
Development of Public Health Research Strategies: The NDSI-IEM is developing research approaches that are suitable for the study of all rare disorders and is evaluating the safety and efficacy of dietary interventions for IEM. These activities fit into the broader context of public health because they will likely lead to better approaches to manage diseases that affect millions of people.
Communications and Outreach: NDSI-IEM staff have given presentations or presented posters on the initiative at the 2011 and 2012 annual meetings of the Society for Inherited Metabolic Disorders, two meetings of the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children in 2012, the 2012 annual meeting of the National Organization of Rare Disorders, the 2013 annual meeting of the American College of Medical Genetics and Genomics [82], the 2013 Abbott Nutrition Metabolic Conference [83], and a 2013 meeting of the Federal Working Group on Dietary Supplements. In addition, NDSI-IEM staff published an article on the role of pediatricians in the nutritional management of IEM [84]. In late 2013, the NDSI-IEM created a new Web portal for researchers, clinicians, patients with IEM, and families that provides links to available resources [85]. NDSI-IEM staff will continue to lead workshops on IEM research at professional meetings.
C. Research Tools
C1. Analytical Methods and Reference Materials Program
Contact: Joseph Betz, Ph.D., [email protected]
Introduction
Analytical methods are used for the quantitative and qualitative analysis of both dietary supplement raw materials and finished products. They generate reliable, accurate data for use by manufacturers, regulators, and researchers to characterize these materials and products. Similarly, certified reference materials (CRMs), including the National Institute of Standards and Technology’s (NIST’s) Standard Reference Materials® (SRMs), are needed for the development, use, calibration, and evaluation of the methods. Validated analytical methods and CRMs ultimately allow analysts to demonstrate that measurements are accurate, precise, and reproducible, thus enhancing consumer confidence in the quality of marketed dietary supplements and ensuring confidence in scientific research results.
The major activities of the ODS Analytical Methods and Reference Materials (AMRM) program are:
- Methods development
- Methods validation
- Reference material development
- Education and outreach
- Evaluations by expert panels
- website reorganization
Activities
Methods Development: This program component focuses on developing and expanding the availability and use of validated, reliable, and accurate analytical methods for quantitative and qualitative characterization of specific dietary supplements and their ingredients.
- Interagency Agreement with U.S. Department of Agriculture (USDA): ODS provided funding to the USDA's Food Composition and Methods Development Laboratory [86] to support the development of techniques to verify the identity of botanicals. This agreement led to articles published in 2010–2013 describing candidate methods for determining the identity and/or provenance of:
-
- Panax (ginseng) [87-89]
- Green tea [88, 90, 91]
- Mustard greens [92]
- Grapefruit [93]
- Other botanicals or botanicals in general [94-99]
Methods Validation: The goal of methods validation work is to produce, and make available, analytical methods that have been shown by rigorous scientific evaluations to be accurate, precise, and reliable.
Reference Material Development: The goal of this work is to produce, and make available, SRMs that are appropriate for analytical methods development, validation, and demonstration of laboratory proficiency. These SRMs can also be used for basic, preclinical, and clinical studies on the biological effects of dietary supplements in health and disease.
The activities of this part of the AMRM program focus on three types of reference materials: biological reference materials for botanical identity confirmation, single pure constituents used for quantitative analysis and confirmation of analyte identity, and matrix reference materials used to evaluate analytical methods and laboratory performance.
Education and Outreach: This activity focuses on the creation and dissemination of information and data about validated analytical methods and reference methods in the peer-reviewed scientific literature. This activity is also designed to expand the use of these methods by the informed ODS stakeholder community and other interested parties.
Activities include AMRM program staff publications; presentations at scientific conferences and other stakeholder events; and participation by AMRM program staff in activities requiring staff expertise, such as those associated with USDA's Dietary Supplement Ingredient Database project and development of the Dietary Supplement Label Database.
The following publications have resulted from this work:
- Interagency Agreement with the U.S. Food and Drug Administration (FDA): This agreement, which was in place from 2002 to 2009, supported an infrastructure contract with AOAC International. Through a second ODS contract, AOAC International is prioritizing 25 ingredients for analytical methods validation between 2013 and 2018. To date, AOAC International has prioritized seven ingredients (chondroitin, phosphodiesterase-5 inhibitors, anthocyanins, ashwagandha, folin-c, cinnamon, and kratom).
Agreement with the National Center for Complementary and Integrative Medicine (NCCIM; formerly called the National Center for Complementary and Alternative Medicine [NCCAM]) for Analytical Methods Validation: NCCAM and ODS formed a partnership to provide supplemental funding (using a National Institutes of Health [NIH] administrative supplement mechanism [100]) to existing NCCAM grantees.
Under this partnership, ODS and NCCAM co-funded five administrative supplements in 2010–2013 for validation studies of analytical methods for constituents of natural products developed by investigators with active NCCAM-supported research grants or other funding mechanisms. The parent grant projects involved research on:
-
- Fatty acids in serum [101]
- Tarragon (Artemisia dracunculus)
- Carotenoids
- Metabolites of polyphenols, the most common antioxidants in the diet, blood, and brain tissue
- Silymarin (from milk thistle, Silybum marianum)
- Sutherlandia frutescens, an African plant used in traditional medicine
- Hop prenylflavonoids in human serum [12]
- This partnership also funded training for investigators performing biomedical research on botanicals investigated in their parent grants. The original notice for supplemental funding was reissued in 2013 with receipt dates through 2015 and with new NIH partners (National Cancer Institute; National Institute of Diabetes and Digestive and Kidney Diseases; and National Heart, Lung, and Blood Institute).
Funding for Interagency Agreements with the FDA and USDA: This funding supported validation studies that resulted in the following publications:
-
- Single-laboratory validation studies for:
- Catechins and caffeine in green tea [102]
- Phenolic constituents of echinacea [103, 104]
- Ginsenosides in ginseng [105]
- Natural toxins, such as mycotoxins and pyrrolizidine alkaloids, in various botanical raw materials and finished products [106-108]
- Vitamin D3 in dietary supplements [109]
- Multilaboratory validation studies for:
- Lycopene [110]
- Water-soluble vitamins [111]
- Ginsenosides in ginseng [112]
- Development of Guidelines for Validating Botanical Identification Methods: An ODS contract with AOAC International resulted in the publication of guidelines [113] for validating botanical identity methods.
- Interagency Agreement with NIST to Create SRMs: The AMRM program supports an interagency agreement with NIST to create SRMs for constituents of dietary supplement raw materials (including nutrients) and finished products and for nutrient biomarkers in biological matrices (tissues measured, such as blood or saliva). In parallel, ODS supports the development and implementation of laboratory quality assurance programs for supplements and for nutrient and nutrient biomarker measurements in clinical specimens.
Current activities through this agreement include:
-
- Reissuance of the NIST SRM for measuring the products of vitamin D metabolism (needed because the original vitamin D SRM sold out)
- Development of an SRM and reference measurement procedure for iodine and biomarkers of iodine status in urine
- Creation of dietary supplement SRMs, including SRMs for botanicals and nutrients
- Creation of botanical identity reference materials with certified values for DNA fingerprints
- Laboratory quality assurance programs for measuring the metabolic products of vitamin D and of omega-3 and omega-6 fatty acids in human serum
- Pilot program to develop tools for determining the contribution(s) of food sources of vitamin D metabolites to vitamin D status
- Dietary Supplement Laboratory Quality Assurance Program, a no-fee program administered by ODS in partnership with NIST. This program measures concentrations of active and/or marker compounds and nutritional and toxic elements in both "practice" and "test" materials. Performance reports and certificates of completion are provided to participating laboratories to demonstrate compliance with current Good Manufacturing Practices (GMPs) as defined by the FDA. This program also holds workshops to discuss measurement results and advances in methodologies for characterizing dietary supplements. Between 2007 and 2010, more than 40 laboratories participated in five exercises to analyze dietary supplement components in more than 20 blinded samples [114].
- Recent publications from the program include:
-
- Overviews of the quality assurance programs that NIST and ODS have developed to support nutritional measurements [115, 116]
- Characterizations of the following SRMs:
- Berries [117-119]
- Fatty acids [120]
- Ginseng [121]
- Green tea (Camellia sinensis) [90, 122, 123]
- Infant and adult nutritional formulas [124, 125]
- Minerals [126, 127]
- Multivitamin/multielement tablets [128, 129]
- Vitamin D [130-134]
- Overview of laboratory quality assurance programs [116]
- Overview of SRMs for dietary supplements [115]
- A scientific paper evaluating commercial DNA extraction kits for utility in correctly identifying plants used in dietary supplements [135]
- A technical guidance document for validation studies to identify botanical ingredients in dietary supplements [113]
- A textbook [136] providing microscopic descriptions of medicinal plants. In 2012, this textbook received the American Botanical Council's James A. Duke Book Award, which recognizes books or book services that make significant contributions to the medical plants field.
- Publications on measurement methods for:
- Flavonols and polyphenols [137]
- Vitamin D [138]
- Yohimbe [139]
- Other nutrients [125, 140, 141]
- Natural toxins [142-148]
Expert Panel Evaluation: An external expert review panel evaluated the AMRM program in 2012, and the panel expressed enthusiasm for the program's continuing progress and direction. Panel members agreed that the AMRM program's NIST reference material production and quality assurance programs (including education) are its most important accomplishments and the program has had a major impact on the dietary supplement and academic research communities. The panel recommended that the AMRM program take a leadership role in selecting and prioritizing the ingredients and analytes that need reference materials and validation methods and in reviewing candidate validation. Another recommendation was for ODS to expand its administrative supplement program.
Web-Site Reorganization: In September 2014, ODS launched a new version of the AMRM program's Web pages on the ODS website. The new Web pages are easier for stakeholders to navigate. They also include a searchable database of analytical methods.
Future Activities
The expert panel and other stakeholders indicated that funding to develop new methods for raw materials and finished products is not needed, but programs and materials are needed to demonstrate that available methods are suitable for their intended use. The AMRM program published a competitive request for proposals for the development and administration of infrastructure support activities to accomplish this recommendation in FedBizOps (the U.S. government's procurement website) in July 2013. The AMRM program issued a competitive award for this activity in September 2013. AMRM staff will use the panel's recommendations to plan future activities.
The AMRM program has funded validation studies for existing methods (for example, to measure curcuminoids in turmeric, monosulfonylmethane, and silymarins in milk thistle) selected by AOAC International in previous years. The AMRM program will identify the subjects of new validation study projects under the infrastructure support contract and will fund these validation studies.
C2. Dietary Supplement Databases
Contacts:
Johanna Dwyer, D.Sc., R.D., [email protected]
Karen Regan, M.S., R.D., [email protected]
Introduction
ODS has funded and assisted in the development of two primary databases that describe the composition of many dietary supplements offered for sale in the United States. The Dietary Supplement Label Database (DSLD) provides product information taken directly from supplement labels, and the Dietary Supplement Ingredient Database (DSID) provides analytically derived estimates of the amounts of ingredients in selected dietary supplements. When these databases are used with food composition databases, the total daily intakes of nutrients and other bioactive substances from both foods and dietary supplements can be estimated.
Another ODS-supported database, Computer Access to Research on Dietary Supplements (CARDS), contains information on research projects pertaining to dietary supplements funded by the U.S. Department of Agriculture (USDA), Department of Defense, or National Institutes of Health (NIH) [1] since 1999. ODS also provided support for the establishment and growth of other databases containing information on dietary supplements, including those at the University of Hawaii Cancer Center and the University of Minnesota.
Activities
Dietary Supplement Database Working Group: This group advises ODS on developing, launching, and expanding the DSID and DSLD as well as other related database tools. The group consists of representatives from the National Institutes of Health (ODS, National Library of Medicine [NLM], and National Cancer Institute [NCI]), U.S. Department of Agriculture (Agricultural Research Service), Centers for Disease Control and Prevention (National Health and Nutrition Examination Survey), Food and Drug Administration, Department of Defense (DoD), and Department of Commerce (National Institute of Standards and Technology). The working group has met monthly since 2004.
Dietary Supplement Ingredient Database (DSID): The DSID provides estimated levels of ingredients in dietary supplement products sold in the United States [149]. It was developed by the Nutrient Data Laboratory of the USDA's Agricultural Research Service in collaboration with, and with funding provided by, ODS. Intended primarily for research applications, the DSID provides access to analytically derived estimates of ingredients in multivitamin/multimineral dietary supplements for adults and children. These data are summarized by ingredient level across representative products rather than for individual products in the market.
Mean percent differences between analytical and labeled amounts have been determined for each of the vitamins and minerals analyzed. The data are more appropriate for use in population studies assessing nutrient intakes than for assessing specific multivitamin/multi-element supplement products in the marketplace. Representative products can be compared to a user's recommended intake and upper safe level of intake for these nutrients using Web-based calculators.
Analyses of omega-3 and fish oil supplements have been completed, and results will be reported in a coming release of DSID. ODS is now compiling analytic data on prenatal dietary supplements sold over the counter and is updating adult multivitamin-multimineral supplement values.
During the current strategic planning period, the ODS Dietary Supplement Database Working Group developed criteria for choosing botanicals and other dietary ingredients that do not have daily values but should be analytically evaluated and added to the DSID because of public health interest. ODS applied the criteria to the top 11 of 38 commonly used dietary ingredients without daily values that had the highest significance to public health and feasibility of conducting chemical analyses of products containing them.
The working group selected dietary supplements containing green tea as the first ingredient for analysis and inclusion in this DSID initiative. ODS and the Dietary Supplement Database Working Group are working with USDA and outside experts to develop a statistical sampling frame for obtaining green tea supplement products for analysis, and samples are now being collected. ODS began chemical analyses for the pilot green tea study in 2014.
Since the database became available in 2010, the DSID has had 178,645 unique visitors (average 3,308/month) and 215,642 visits: (average 3,993/month).
Dietary Supplement Label Database (DSLD): The DSLD, a joint project of ODS and NLM, lists the full label contents of dietary supplement products marketed in the United States and maintains an archive of products and formulations. The database is of value to research scientists, health care providers, and consumers who want to identify and compare products of interest. The DSLD includes more than 30,000 labels to date, voluntarily submitted by companies, and will eventually incorporate most of the estimated 85,000 supplement products available.
The DSLD has received favorable attention from the media and trade associations. Several supplement manufacturers have volunteered their products for inclusion in the database. The DoD has asked for further consultations with ODS about the possibility of adapting the DSLD to include adverse events for use with warfighters. Since it was launched in 2013, the DSLD has had 116,175 unique visitors (average 7,261/month) and 249,866 visits (average 15,617/month).
The DSLD received national attention on July 10, 2014, when The Vitamin Shoppe, a national retailer of dietary supplements, publicly announced plans to submit all labels of its proprietary line of supplements to the DSLD and that it expected all its vendors to submit their product labels to the DSLD as well by the end of 2015.
Recently, the Dietary Supplement Database Working Group adapted the specifications of LanguaL™, a structured vocabulary for describing each supplement in the database, to conform to U.S. laws and regulations for labeling dietary supplements. LanguaL will facilitate the linking of the DSLD, other supplement databases, and the USDA's food composition database. An article on the use of Langual for classifying and retrieving information in dietary supplement databases has been published [150]. ODS researchers recently prepared an article on caffeine in energy drinks and dietary supplements in the United States using information from the DSLD [151].
ODS collaborates with a database developer working with the European Food Information Resource Network (EuroFir) to ensure that the DSLD and the EuroFir supplement databases are harmonized. EuroFir is a nonprofit international association that supports the use of existing food composition data and resources through cooperation and harmonization of data quality, functionality, and global standards. This collaboration will make it easier to compare data on supplement use in the United States and Europe in the future.
ODS is updating the DSLD in the following ways to improve its functionality:
- Code products using the LanguaL structured vocabulary
- Archive data on products no longer on the market
ODS is adding label information from dietary supplements of particular public health interest to the DSLD in the following priority order:
- Labels collected in the NHANES
- Labels of products sold in the DoD's Post Exchange (PX) stores on military bases
- Labels of products of interest to other federal agencies (e.g., energy supplements)
- Labels of highly fortified food products labeled as dietary supplements
Dietary Supplement Label Database and Dietary Supplement Label Database Outreach: In 2011 and 2014, ODS staff published articles (including one in a high-impact journal) on these databases [150, 152]. Between 2010 and 2014, ODS and its collaborators gave presentations on using the DSID and/or DSLD at more than 28 different national meetings to health professionals, industry representatives, educators, and others. These meetings included the annual National Nutrient Database meeting, NCI's Nutrition and Cancer Practicum, Experimental Biology, ODS Mary Frances Picciano Dietary Supplement Research Practicum, American Academy of Nutrition and Dietetics annual meeting, and AOAC INTERNATIONAL annual meeting. ODS staff used these opportunities to solicit recommendations from meeting participants for additional ingredients to include in the DSID. ODS staff and members of the Dietary Supplement Database Working Group continue to prepare abstracts, posters, presentations, and articles for professional journals.
Computer Access to Research on Dietary Supplements (CARDS): The CARDS database contains information on research projects pertaining to dietary supplements funded by the USDA, DoD, or NIH [153] since 1999. CARDS users include scientists, industry representatives, health care professionals, the general public, federal agency staff, and the U.S. Congress.
CARDS data come from the Human Nutrition Research and Information Management (HNRIM) system, an online federal government database created for fiscal accounting, management, and control of cross-agency nutrition research activities. Because CARDS has a narrower focus than HNRIM, CARDS provides more details on each research project. For example, CARDS identifies the dietary supplements or ingredients studied, health outcomes measured, and research methodology used. A CARDS search can quickly identify federally funded research related to specific dietary supplements, populations, and outcomes.
ODS researchers used CARDS data to describe NIH and USDA dietary supplement research funding in 1999–2007 [154] and the NIH dietary supplement research portfolio in 2009–2011 [5].
- Participation in the Interagency Committee on Human Nutrition Research (ICHNR): After some years of inactivity, the ICHNR was recently reconstituted. The committee's goal is to enhance coordination of federal agencies engaged in nutrition research. The ICHNR helps ensure that the nation benefits from focused, coordinated human nutrition research and the results provide clear information on which to base dietary guidance for Americans.
HNRIM was originally developed under the auspices of the ICHNR. ODS anticipates that ICHNR's reactivation will facilitate the collection of federally funded nutrition and dietary supplement research data in a more timely manner.
Flavonoid Database Project: Flavonoids are polyphenolic phytochemicals found in almost all plants, many foods, and some dietary supplements. With funding from ODS, USDA is adding data on the content of six classes of flavonoids in supplements and foods to the database it maintains for assessing intakes from participants in NHANES. The Dietary Supplement Database Working Group reviewed specifications for this addition in 2013. Several members of the working group jointly presented flavonoid intake analyses in the most recent release of the NHANES data.
Database Development at the University of Hawaii Cancer Center and the University of Minnesota: Over the past decade and with partial funding from ODS, the University of Hawaii Cancer Center developed a database of supplements used in research studies on its "multi-ethnic cohort"; this database is available for public use. ODS also funded the University of Minnesota to develop an optional dietary supplement module to accompany its dietary analysis software for food intakes. The module is available for purchase and provides estimates of total nutrient intakes from foods and supplements.
PubMed Dietary Supplements Subset: ODS developed this subset in collaboration with NLM. This resource, which has been available since December 2010, limits PubMed search results to citations from the dietary supplement literature [155]. ODS Communications Program staff works with NLM to update the search strategy for defining this subset for the annual PubMed update each December. This dataset replaces the International Bibliographic Information on Dietary Supplements (IBIDS) database, which ODS and the USDA maintained between 1999 and 2010, and the ODS Annual Bibliography of Significant Advances in Dietary Supplement Research through 2010.
Future Activities
ODS will continue to expand the DSID to include estimated levels of ingredients in prenatal vitamins sold over the counter and omega-3 fatty-acid products. ODS also plans to analyze additional botanicals and other products and add these data to the DSID as priorities and finances permit.
ODS will explore the feasibility of linking the DSLD to the following software applications of federal partners:
- NCI's ASA24TM 24-hour recall data-collection tool and Food Frequency Questionnaire to allow NCI to add many more supplement labels to ASA24 and enhance this excellent research resource
- NHANES to reduce the amount of time that NHANES colleagues spend gathering labels for the NHANES survey database and identifying supplements taken by NHANES participants, which will also decrease the need for ODS to provide funding for this purpose
ODS plans to add data from the DSLD to the dietary supplement database being developed by the Food and Agricultural Organization of the United Nations. This effort will prevent duplication of effort and help harmonize dietary supplement databases on an international level.
ODS will also encourage dietary analysis software vendors to include the DSLD in their proprietary products. At present, it is difficult to integrate the DSLD with food composition tables and dietary analysis software. Once the capacity to download the entire DSLD is available, ODS will welcome researchers, international organizations and vendors, and others to add the DSLD or parts of it to their databases.
If resources permit, ODS will sponsor a meeting of experts from government, academia, and the private sector in early 2015 to review progress in developing the DSID and DSLD as well as future directions for the development and use of dietary supplement databases. ODS will invite USDA to cosponsor the workshop.
ODS will redesign CARDS in 2015 to take advantage of new data management and reporting technologies. The new database design will include many more data fields, enabling CARDS to provide more flexible data reporting and portfolio analysis. The redesigned site will also provide links between funded research projects and associated clinical trials information and publications. CARDS currently provides data on projects funded between 1999 and 2010. Once the database redesign is complete, ODS staff will enter data on projects funded in 2011 and 2012 to CARDS. In the interim, the data are available by request.
Planned database-related projects are as follows:
- Continue to collaborate with colleagues at USDA, CDC, and NIH in 2015 to study the associations between flavonoid intakes from food and supplements and both total and cause-specific mortality in the NHANES 1988–1994 cohort
- Compare relative risk estimates of the associations between total dietary intake and mortality from estimates using flavonoid intake data derived from different databases (e.g., for the incomplete version from the early 2000s and the more complete database of 2013)
- Explore whether development of software and infrastructure that links the DSID and DSLD to validate label claims for some products in the DSLD is necessary and feasible
- Assess the feasibility of incorporating labels of supplements for treating rare diseases into the DSLD
- Refine the DSLC user interface and underlying search engine to help users find, sort, and compare label information from multiple products more quickly and easily
- Add a glossary of synonyms for names of nutrients and other ingredients to simplify searches and indexing the DSLD
- Provide download capability so that the data in the DSLD can be integrated into other software
- Add links from the DSLD to MedlinePlus®, PubMed®, and NIH fact sheets
- Harmonize the DSLD with the USDA's food composition database to facilitate estimates of total dietary intake.
C3. Evidence-Based Review Program
Contact: Anne L. Thurn, Ph.D., [email protected]
Introduction
Systematic reviews provide independently conducted, comprehensive, and objective assessments of available information addressing precise questions. These reviews represent a rigorous and transparent approach for synthesizing scientific evidence that minimizes bias. The medical community uses these reviews to support development of clinical and public health practice guidelines, set research agendas, and formulate scientific consensus statements.
ODS has developed an evidence-based review program using the Evidence-based Practice Center (EPC) program established by the Agency for Healthcare Research and Quality (AHRQ). To date, this ODS program has sponsored 17 evidence reports on a range of supplement-related topics, including B-vitamins, ephedra, multivitamin/mineral supplements, omega-3 fatty acids, soy, probiotics, and vitamin D. A complete list of ODS-sponsored evidence reports and related publications is available on the ODS website [156].
Activities
AHRQ Systematic Reviews: Since 2010, ODS has sponsored two published AHRQ systematic reviews:
- Vitamin D and Calcium: A Systematic Review of Health Outcomes (Update) [17]: Data from nearly 250 new studies did not alter the conclusions about vitamin D and health from an earlier AHRQ systematic review. Despite the abundance of newer research, the authors of this updated review were unable to specify a relationship between vitamin D and health outcomes other than bone health. This report provided background information for speakers and panel members at the NIH vitamin D and primary care conference on December 2–3, 2014 [31].
- Safety of Probiotics Used to Reduce Risk and Prevent or Treat Disease: There is a lack of assessment and systematic reporting of adverse events in probiotic intervention studies, and interventions are poorly documented. The available evidence in randomized, controlled trials does not indicate an increased risk. However, rare adverse events are difficult to assess. Despite the substantial number of publications, the current literature is not well equipped to answer questions on the safety of probiotic interventions with confidence [157].
ODS is sponsoring additional systematic reviews to update two reports on omega-3 fatty acids—one focused on cardiovascular disease outcomes and the other on early cognitive development.
AHRQ Technical Reports Related to Nutrition: Since 2010, ODS has sponsored the development of six technical reports through the AHRQ EPC program [158-163]. The purposes of these reports were to:
- Identify the challenges, advantages, and limitations of conducting nutrition-based systematic reviews
- Work with a panel of experts to explore approaches for integrating systematic reviews into processes used to derive dietary reference intakes
- Evaluate the compliance of currently available nutrition-related systematic reviews with generally accepted quality guidelines for evidence-based reviews
- Critically explore the consistencies and inconsistencies in results between observational and intervention studies on nutrients
Findings included:
- Identification of the steps for generating systematic reviews, including those unique to the field of nutrition
- Identification of essential nutrition-related considerations for performing systematic reviews in the field of nutrition
- Determination that systematic reviews can be productively used to inform the development of reliable dietary reference intakes
- Determination that the reporting quality of systematic reviews in general has improved after the publication of systematic review reporting standards [164], but the reporting of nutrition variables has not. This finding has encouraged researchers to improve their adherence to consensus methods and reporting standards to improve the usefulness of systematic reviews on nutrition topics.
- Determination that either of two methods for identifying signals for assessing the need to update an evidence review can be used for this task, providing options for researchers using systematic reviews to inform their work
These reports resulted in three publications in the peer-reviewed literature [162, 163, 165] that will help in the assessment of the current state of the science.
D. Collaborations with Other Federal Agencies
D1. Federal Working Group on Dietary Supplements
Contact: Paul R. Thomas D.Sc., [email protected]
Activities
Federal Working Group on Dietary Supplements: Members represent the National Institutes of Health and other federal agencies. This group has met twice a year since January 2005 to strengthen individual and collaborative efforts involving dietary supplement research, education, and communication.
D2. ODS Collaborations with the U.S. Department of Agriculture (USDA)
Contact: Paul M. Coates, Ph.D., [email protected]
Introduction
ODS collaborates with USDA's Agricultural Research Service (ARS) in Beltsville, Maryland, on several projects.
Activities
Dietary Supplement Ingredient Database (DSID): For 10 years, ODS has collaborated with USDA and funded the development of this database to provide analytically derived values for nutrients in certain classes of dietary supplements based on chemical analysis. Such methods allow analysts to show that their measurements are accurate, precise, and reproducible. Recent work on this database has focused on multivitamin-multimineral products. ODS is now collecting analytically derived values for green tea-containing dietary supplements.
Development of Spectral Fingerprinting Methods for Rapid Characterization and Authentication of Botanical Dietary Supplements: Correct identification of ingredients in botanicals (herbs and other plant components) is necessary for reproducible research and is required by the U.S. Food and Drug Administration to market products in the United States. Because processed ingredients are difficult to identify by sight, this project is exploring and developing methods to identify botanicals using spectroscopic fingerprinting. The project will develop methods to collect spectra, assess the reliability of the techniques, and develop an electronic plant fingerprint archive for each botanical for future reference. This project, which began before the period of the current strategic plan, will continue indefinitely.
Development and Validation of Methods for Toxic Dehydropyrrolizidine Alkaloids (PAs) in Dietary Supplements: Some botanicals used in traditional medicine contain natural poisons. PAs, for example, can damage the liver and cause cancer or birth defects. Dozens of unique PAs are present in hundreds of plant species, but there are no validated analytical methods to characterize and measure them. The USDA's Poisonous Plant Research Laboratory has unique expertise with these poisons and is creating and publishing validated analytical methods to measure them. This work began in 2011 and will continue until approximately 2016, when ODS will provide PA standards and methods to assess for PAs in the popular herbs comfrey and butterbur to the National Institute for Standards and Technology so that it can produce Standard Reference Materials®.
Cocoa Studies: Consumption of cocoa has been linked with health benefits in studies in humans, probably because it contains polyphenols, which might have a role in preventing certain diseases. Several studies suggest that microbes in the intestines help metabolize cocoa polyphenols and contribute to the beneficial effects of these compounds. Moreover, cocoa polyphenols may promote the growth of beneficial microbes. This project is using pigs to assess the activity in the intestines of different cocoa polyphenols in combination with Bifidobacterium and Lactobacillus species of microbes and the impact of these combined changes on overall health. The first study, begun in the fall of 2013, showed that cocoa can alter microbes. A second study, designed to learn whether cocoa can increase the efficacy of probiotics (again using Bifidobacterium and Lactobacillus species), which promote the growth of beneficial bacteria in the digestive system, will begin in early 2015.
John A. Milner Postdoctoral Fellows Program: A joint activity of the USDA Beltsville Human Nutrition Research Center (BHNRC) and ODS, this new opportunity that began in 2014 enables postdoctoral fellows to conduct research on components in foods and dietary supplements that affect health and learn about the translation of nutrition science into policy. ODS and the BHNRC have joined to honor Dr. Milner, who directed BHNRC until his death, by encouraging the careers of junior scientists in the field.
D3. Department of Defense–ODS Nutrition Collaboration
Contact: Rebecca B. Costello, Ph.D., [email protected]
Introduction
Approximately 50% of soldiers and civilians alike take at least one dietary supplement per week. However, soldiers are more likely than civilians to take several types of supplements at the same time and to use protein and amino acid supplements.
ODS assists the Department of Defense (DoD) in studying the use, safety, and efficacy of dietary supplements in military populations and in the department's efforts to educate these populations about the benefits, risks, and proper use of such products. ODS provides expert consultations to DoD, serves on DoD advisory committees, and co-funds research on issues of common interest to ODS and DoD. This collaboration keeps ODS informed of DoD activities related to dietary supplements.
Activities
Research
Evidence-based Reviews: An ODS staff member has served as a consultant on evidence-based reviews prepared by the U.S. Pharmacopeia, with support from DoD, of the benefits and safety of three amino acids (beta-alanine, L-arginine, beta-alanine) used in combination with caffeine and/or creatine to improve athletic performance.
Study of the Effects of Caffeine and Heat Exposure: ODS co-funded a grant with the U.S. Army to the Uniformed Services University of the Health Sciences (USU). This grant supports a study on the effect of caffeine and heat stress on health. The study will also identify and evaluate the characteristics of individuals who are particularly susceptible to adverse effects from this combination. Results are expected in 2015.
Omega-3 Fatty Acid Feeding Study: ODS provided consultation for an omega-3 fatty acids study funded by DoD and the Samueli Institute. This study is evaluating the effects on physical and mental performance of food products that are highly enriched with omega-3 fatty acids.
Education
Military Participation in the ODS Mary Frances Picciano Dietary Supplement Research Practicum: ODS has provided education to an average of five military health-care professionals for each of the past eight years at its annual Dietary Supplement Research Practicum. In addition, several members of the military have delivered presentations as part of the practicum.
Web-Based Tutorials: ODS provided funds for, and staff members served as reviewers of, Web-based tutorials on dietary supplements for warfighters and military health-care providers. The University of North Carolina at Chapel Hill developed these tutorials through a DoD contract. The USU Human Performance Resource Center website [166] makes these new resources available to military personnel and the general public.
Operation Supplement Safety: ODS staff provided input into the DoD plan for, and launch of, Operation Supplement Safety [167]. This is an educational initiative aimed at service members, retirees, family members, health-care providers, and DoD civilian employees. The initiative provides information about dietary supplements and how to choose them wisely.
Guidance on Implementing Recommendations
Guidance on Implementing Institute of Medicine Recommendations: ODS worked with USU to ensure that the military meets all 12 recommendations from the 2008 Institute of Medicine (IOM) report, Use of Dietary Supplements by Military Personnel [168]. In particular, ODS staff provided input to help DoD standardize the instruments and questions employed in the department's surveys of health-related behaviors and dietary supplement use by military personnel. ODS also provided input on Web-based tutorials (described above) on dietary supplements in response to the IOM recommendations.
Information Sharing Among Federal Agencies
DoD Dietary Supplement and Other Self-Care Products Subcommittee: An ODS staff member is a member of the DoD Dietary Supplement and Other Self-Care Products Subcommittee of the DoD Nutrition Committee. The subcommittee includes representatives of all branches of the military, Food and Drug Administration, and Department of Veterans Affairs. This group produces educational materials on dietary supplements, conducts military-specific research, monitors and reports on adverse events related to dietary supplements, and identifies research gaps and opportunities to share resources and contain costs.
DoD Center Alliance for Dietary Supplement Research: ODS is represented on the DoD Center Alliance for Dietary Supplement Research, a collaborative effort between investigators at the U.S. Army Research Institute of Environmental Medicine and USU. Its mission is to study and monitor the use, adverse events, and safety and efficacy of the dietary supplements used by the U.S. military.
Federal Working Group on Dietary Supplements: Through this working group, representatives from ODS, other components of the National Institutes of Health, DoD, and other federal agencies meet to share information and discuss issues, initiatives, and research related to dietary supplements. This group has met twice a year since January 2005.
D4. Dietary Reference Intake (DRI) Initiative
Contacts:
Christine Taylor, Ph.D., [email protected]
Elizabeth Yetley, Ph.D. [email protected]
Introduction
ODS supports the development of nutrient reference values (known as dietary reference intakes [DRIs]) as well as research related to the science underpinning these reference values and their application.
Activities
Updating a Review of the Health Effects of Vitamin D: ODS was the primary funder of a 2009 evidence-based review of the health effects of vitamin D and calcium by the Agency for Healthcare Research and Quality (AHRQ). This review's purpose was to inform the deliberations of an Institute of Medicine (IOM) panel of scientists that evaluated the DRIs for these two nutrients. In 2013, ODS contracted again with AHRQ to update the 2009 review, taking into account studies published through 2013 related to the health outcomes associated with vitamin D alone or in combination with calcium. The authors of the updated review, issued in September 2014, concluded that despite the availability of new research, data remained inconclusive regarding vitamin D's health benefits other than for bone [17]. Therefore, the findings that the IOM panel used remain up to date. This information is useful to the IOM as it considers its options for dealing with the body of data related to vitamin D and health. See section on the ODS Evidence-Based Review program for more details.
Funding the Deliberations of IOM Expert Panel on DRI: An earlier document developed by ODS and other government agencies [169] summarized the evidence to support a review of vitamin D and calcium relative to updating the existing DRIs. When the IOM convened an expert panel in 2009 to update the DRIs, ODS provided funding, as did other agencies of the U.S. and Canadian governments, to support this work. The IOM issued the final DRI report for vitamin D and calcium in late 2011 [16].
Federal Government Consideration of Nutrients for Next DRI Review: The U.S. and Canadian governments have established a joint steering committee to identify DRI needs and coordinate government sponsorship of DRI reviews. In 2013, the committee recognized the importance of input from individuals and organizations for prioritizing decisions about funding future DRI reviews. The committee implemented a nomination process using a Department of Health and Human Services website and compiled the submissions for review [170]. ODS provided staff support for evaluating and ranking of the nominated nutrients.
Proposal for Workshop on Chronic Disease as a Basis for Nutrient Reference Values: The challenges of using reduction of chronic disease risk as the basis for a nutrient reference value have led to calls for a government-sponsored workshop to elucidate options for such an approach. ODS, in cooperation with Canadian colleagues, developed a proposal for such a workshop and submitted it for consideration to the DRI steering subcommittee is planned for spring 2015.
Research on Use of DRIs to Assess Nutrient Status: The IOM has established a statistical approach to assess the nutrient status of a population based on estimates of nutrient intake. ODS supported research on the ability to use this approach based on a biomarker measure [23].
E. Communications
E1. Communications Program
Contacts:
Anne L. Thurn, Ph.D., [email protected]
Paul R. Thomas, Ph.D., [email protected]
Claudia D. Faigen, M.A., [email protected]
Introduction
The ODS Communications Program includes a broad spectrum of out¬reach activities, such as enhancing and maintaining the ODS website and social media; responding to media and public inquiries; and developing up-to-date, useful information for consumers and health professionals [171].
Activities
ODS Publications and Other Resources
Fact Sheets: Communications Program staff prepare fact sheets on ingredients (primarily nutrients) in dietary supplement products. Twenty-seven health professional fact sheets are currently available on the ODS website [172]. All of the dietary supplement fact sheets, which have a similar format, are scientifically detailed, fully referenced, and updated as necessary. Two scientific experts in nutrition and dietary supplements are responsible for revising and updating the ODS fact sheets.
During the current strategic planning period, ODS added two new consultants to speed up the writing of fact sheets, and streamlined the processes for preparing, reviewing, and updating them. Other notable accomplishments include:
- Since 2010, the Communications Program has updated most of its fact sheets.
- ODS began posting consumer versions of these fact sheets in January 2010. These documents provide some of the same information as the health professional fact sheets but are shorter and easier to read, present information in a more helpful way, and are appropriate for both health-conscious consumers and busy health professionals who desire a "just-the-facts" approach. Fourteen consumer fact sheets are now available on the ODS website.
- ODS translated all 14 of its consumer fact sheets into Spanish.
- In 2010, ODS began making more frequent updates to its fact sheets to include important new research results and authoritative advice.
- ODS recently began developing topic-specific fact sheets and posted its first fact sheet in this category in the summer of 2012 [173]. This fact sheet, on multivitamin/mineral supplements, is available in three versions; one for health professionals, a consumer version in English, and a consumer version in Spanish. ODS has also developed a fact sheet on dietary supplements for weight loss that it posted on its website in October 2014 [174].
What You Need to Know Brochure: Since 2010, the Communications Program has revised its Dietary Supplements: What You Need to Know brochure and translated it into Spanish. The program is now developing a lower-literacy version of this brochure.
Newsletters and E-mail Blasts: The Communications Program disseminates news about dietary supplements research to a broad range of audiences through the following mechanisms:
- ODS Listserv: In December 2010, the Communications Program replaced the outdated National Institutes of Health (NIH) listserv with software that offers several new features, including updates on numbers of people who open ODS e-mails and live links in the e-mails.
- ODS Update: This quarterly newsletter for professionals provides information about ODS programs and activities, new products and materials, meetings and exhibitions with an ODS presence, and staff publications and presentations. Since January 2010, ODS has sent 14 issues to the ODS e-mail list of more than 4,200 subscribers and posted these issues on the ODS website [175].
- The Scoop: ODS sends this consumer-oriented newsletter to more than 3,800 subscribers of its listserv. The Scoop provides information readers can use to make informed decisions about taking dietary supplements [175]. ODS has published nine issues since it created this publication in February 2011.
- Special Supplement e-blasts: As needed, ODS sends important announcements by e-blasts to its listserv. ODS has issued 13 e-blasts since December 2010 on such topics as ODS participation in national conferences, upcoming meetings on dietary supplements, and funding opportunities [175].
- News o' the Day: This daily e-mail keeps ODS staff current on news on dietary supplements, nutrition, and related topics collected from such sources as peer-reviewed journals, government documents, newspaper articles, and blog posts.
Videos: In 2014, ODS produced three videos to introduce the office and its website to a broader audience. They are available on the ODS website and the NIH OD YouTube Channel:
Outreach Using Social Media and Mobile Applications: ODS is taking advantage of social media to make its information available to a larger audience than its website reaches. ODS created a Facebook page in March 2012 and posts one message every workday on this page. The ODS Facebook page reaches an average of 9,000 users per month. ODS started its Twitter feed in June 2010 and sends out one to three "tweets" each workday. Currently, ODS has more than 9,000 Twitter followers.
ODS developed the My Dietary Supplements (MyDS) application for the Apple iPhone, iPad, and iPod touch. MyDS provides an easy way to keep track of the vitamins, minerals, herbs, and other products users take; offers access to science-based, reliable information on dietary supplements; and delivers general information about ODS [176]. Users can store the names and amounts of the dietary supplements they take in the app for later use. Users can also e-mail their list of dietary supplements from the app to themselves or a health-care provider. ODS promotes the app through its newsletters, website, and exhibits program.
ODS launched the first version of MyDS in October 2010 and an updated version in February 2012 for many popular devices (including Apple, Android, and BlackBerry devices) and desktop browsers. ODS launched MyDS Español in September 2012.
PubMed Dietary Supplements Subset: Developed in collaboration with the National Library of Medicine (NLM), this resource has been available since December 2010 [155]. The subset limits PubMed search results to citations from the dietary supplement literature. ODS Communications Program staff work with NLM to update the search strategy for defining this subset for the annual PubMed update each December. This dataset replaces the International Bibliographic Information on Dietary Supplements (IBIDS) database, which ODS and the U.S. Department of Agriculture maintained between 1999 and 2010, and the ODS Annual Bibliography of Significant Advances in Dietary Supplement Research through 2010.
Electronic Media
Website: The Communications Program staff maintains and updates the ODS website, which received more than 900,000 visits per month in 2014. The site provides links to many resources, including ODS funding opportunities, a list of all ODS-funded grants, and databases of interest to ODS stakeholders.
During the current strategic planning period, the Communications Program made the following enhancements to the ODS website:
- Added home page billboards that display highlights of ODS programs
- Created a new administrative interface that allows non-technical staff to quickly and easily modify headlines and other pages on the website
- Created pages for the Vitamin D Initiative, Vitamin D Standardization Program, and Nutrition and Dietary Supplement Interventions for Inborn Errors of Metabolism [85,177,178]
- Developed a Spanish-language portal with links to all ODS information available in Spanish [179]
- Revised the frequently asked questions page [180]
- Began using quick response (QR) codes, a type of barcode, on many print publications and ODS scientific meeting posters with links to relevant publications or programs on the ODS website
Since 2011, the Communications Program has developed four non-public websites to share information on the following ODS programs with program members or stakeholders:
- Botanical Research Centers Program
- Federal Working Group on Dietary Supplements
- Nutrition and Dietary Supplement Interventions for Inborn Errors of Metabolism
- Vitamin D Standardization Program
ODS launched a reorganized version of its Analytical Methods and Reference Materials Program section of the ODS website in September 2014. The Web pages are easier for stakeholders to navigate and include a searchable database of analytical methods [58].
Meeting- and Conference-Related Activities
Meeting Exhibits Program: Until the end of FY 2014, ODS exhibited at approximately 9–12 meetings per year. The program has since been put on hold as a cost-cutting measure.
Presentations at Professional Meetings: The Communications Program has custom-designed five PowerPoint templates to help ODS staff prepare professional-quality presentations that will increase awareness of ODS among a broader audience. ODS purchased and maintains a collection of photos for use by staff in PowerPoint presentations. A list of staff presentations is available on the ODS website [181].
Meeting Planning Support: Communications Program staff coordinate all aspects of ODS-sponsored meetings. For example, in 2013, Communications Program staff supported 18 ODS meetings, including maintaining a master calendar of all past and upcoming ODS meetings.
Strategies to Communicate Nutrition News to Other Government Agencies
ODS Working Groups and Committee Memberships: ODS Communications Program staff organize meetings for two federal working groups:
- Federal Working Group on Dietary Supplements: Members represent NIH and other federal agencies. This group has met twice a year since January 2005 to strengthen individual and collaborative efforts involving dietary supplement research, education, and communication.
- Federal Working Group on Vitamin D: This group has a similar structure to the Federal Working Group on Dietary Supplements. This group meets once or twice a year to identify research needed to improve scientific understanding of vitamin D and its role in health, disease prevention, and treatment.
ODS staff, including Communications Program staff, participate in a wide range of trans-NIH and federal working groups and committees that pertain to dietary supplements (e.g., NIH Nutrition Coordinating Committee, NIH Prevention Research Coordinating Committee, and Biomarkers of Nutrition and Development Program).
Communicating Nutrition News across Federal Agencies and to Federal Officials: ODS is asked periodically to prepare documents, respond to questions, or provide background information and interpretation on nutritional matters by federal agencies and officials. For example, in November 2010, ODS prepared summaries and talking points for the Institute of Medicine's report on recommended intakes of vitamin D and calcium for use by spokespeople at the Department of Health and Human Services (DHHS) and NIH. In March 2014, ODS prepared materials for the NIH Deputy Director on the nutritional labeling of foods as a background for his participation in a federal meeting on the subject. ODS also serves as a source of information to the NIH Associate Director for Prevention, who became the point of contact in June 2014 for all NIH interagency issues related to nutrition.
Responses to Media Inquiries
Responses to Media Inquiries: ODS Communications Program staff triage requests from broadcast, print, and online media two or three times per week, on average. Since 2010, a growing number of members of the media have sought reliable resources, such as those of ODS, for their articles on dietary supplements. To keep up with these requests, the Communications Program now provides two staff members with off-site (remote) access to the ODS media request mailbox so that all requests can be answered in a timely way.
Two Communications Program consultants with broad nutrition science expertise prepare ODS staff members for media interviews by providing background materials and assistance with preparing messages and talking points. To ensure that ODS has the expertise available when needed, the Communications Program arranged for these two consultants to receive blanket approval from DHHS to represent ODS in media interviews. Communications Program staff also coach each expert as necessary. For example, in 2012, the Communications Program provided support to ODS Director Paul Coates for a radio interview on the nationally syndicated Diane Rehm Show.
During the current strategic planning period, the Communications Program developed a new media response protocol that includes adhering to the format required for obtaining NIH and DHHS approval for responding to media requests. The protocol identifies several layers of backup to ensure that that ODS provides high-quality responses even when a reporter is working under a short deadline.
Other Outreach
Responses to Public Inquiries: ODS responds to approximately 30 inquiries per month, mostly from consumers, but occasionally from health professionals and industry representatives.
Outreach Assistance: The ODS Communications Program helps ODS staff with outreach (including publicity and Webinars) for their public events, such as the ODS Mary Frances Picciano Dietary Supplement Research Practicum and the Vitamin D Standardization Program. The Communications Program assists with publicity, preparation of materials and presentations, identification of a venue, and arrangement and management of Webinars. The program identifies and collects lists of institutions and people to target with invitations to apply to the practicum. Communications Program staff members regularly review news releases, brochures, and publications for activities that ODS cosponsors with such organizations as the National Institute of Standards and Technology and most recently, the National Library of Medicine.
Evaluation Support
Program Evaluation Support: ODS Communications Program staff oversaw evaluations of IBIDS, the ODS Annual Bibliography of Significant Advances in Dietary Supplement Research, and the Computer Access to Research on Dietary Supplements database in 2010. These evaluations led to the replacement of IBIDS and the bibliography with the PubMed Dietary Supplements Subset. More recently, ODS Communications Program staff members assisted with evaluations of the ODS Mary Frances Picciano Dietary Supplement Research Practicum and Analytical Methods and Reference Materials Program.
References
- Office of Dietary Supplements, National Institutes of Health. Staff Publications. 2014.
- Office of Dietary Supplements, National Institutes of Health. ODS Research Portfolio.
- Clinical Research Forum. Clinical Research Forum
 .
.
- National Institutes of Health. Gut Flora Metabolism of Dietary Phosphatidylcholine and Cardiovascular Disease. Grant Number: 5R01HL103866-03.
- Garcia-Cazarin ML, Wambogo EA, Regan KS, Davis CD. Dietary supplement research portfolio at the NIH, 2009-2011. J Nutr 2014;144:414-8.[PubMed abstract]
- National Center for Complementary and Alternative Medicine (NCCAM). NCCAM Policy: Natural Product Integrity.
- Office of Dietary Supplements, National Institutes of Health. FY2015 Referral Guidelines: The Office of Dietary Supplements (ODS) Research Scholars Program. 2015.
- Office of Dietary Supplements, National Institutes of Health. Botanical Dietary Supplements.
- Office of Dietary Supplements, National Institutes of Health. NIH Botanical Research Centers Program.
- National Institutes of Health. National Center for Complementary and Integrative Medicine (NCCIM).
- National Institutes of Health. National Cancer Institute
 .
.
- Yuan Y, Qiu X, Nikolic D, Dahl JH, van Breemen RB. Method development and validation for ultra-high-pressure LC/MS/MS determination of hop prenylflavonoids in human serum. J AOAC Int 2012;95:1744-9. [PubMed abstract]
- National Center for Complementary and Alternative Medicine (NCCAM). 2013 NIH Botanical Research Expert Panel Meeting: Executive Summary.
- National Institutes of Health. Botanical Dietary Supplement Research Centers (BDSRC) (P50).
- Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Vitamin D and Calcium: A Systematic Review of Health Outcomes. Evidence Report/Technology Assessment No. 183. (Prepared by Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I). AHRQ Publication No. 09-E015. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [PubMed abstract]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D
 . Washington, DC: The National Academies Press; 2011.
. Washington, DC: The National Academies Press; 2011.
- Newberry SJ, Chung M, Shekelle PG, Booth MS, Liu JL, Maher AR, et al. Vitamin D and Calcium: A Systematic Review of Health Outcomes (Update). Evidence Report/Technology Assessment No. 217
 . (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2012-00006-I.) Rockville, MD: Agency for Healthcare Research and Quality; 2014.
. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2012-00006-I.) Rockville, MD: Agency for Healthcare Research and Quality; 2014.
- Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: Measurement procedure issues. Am J Clin Nutr 2011;94:297s-302s. [PubMed abstract]
- Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006
 . NCHS Data Brief 2011:1-8.
. NCHS Data Brief 2011:1-8.
- Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988-1994)
 . NCHS Data Brief 2011:1-8.
. NCHS Data Brief 2011:1-8.
- Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, et al. Dietary supplement use in the United States, 2003-2006. J Nutr 2011;141:261-6. [PubMed abstract]
- Pfeiffer CM, Schleicher RL, Johnson CL, Coates PM. Assessing vitamin status in large population surveys by measuring biomarkers and dietary intake - two case studies: Folate and vitamin D. Food Nutr Res 2012;56. [PubMed abstract]
- Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: A study using vitamin D. Am J Clin Nutr 2013;97:72-8. [PubMed abstract]
- Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140:817-22. [PubMed abstract]
- Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, et al. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr 2014;144:654-9. [PubMed abstract]
- Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, Yetley EA, Looker AC, Schleicher RL, et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab 2013;98:3001-9. [PubMed abstract]
- Kramer H, Sempos C, Cao G, Luke A, Shoham D, Cooper R, et al. Mortality rates across 25-hydroxyvitamin D (25[OH]D) levels among adults with and without estimated glomerular filtration rate <60 ml/min/1.73 m2: The third National Health and Nutrition Examination Survey. PLoS One 2012;7:e47458. [PubMed abstract]
- Durazo-Arvizu RA, Dawson-Hughes B, Sempos CT, Yetley EA, Looker AC, Cao G, et al. Three-phase model harmonizes estimates of the maximal suppression of parathyroid hormone by 25-hydroxyvitamin D in persons 65 years of age and older. J Nutr 2010;140:595-9. [PubMed abstract]
- Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, et al. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: A case study of the program's potential for national nutrition and health surveys. Am J Clin Nutr 2013;97:1235-42. [PubMed abstract]
- Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: National surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32-40. [PubMed abstract]
- Office of Dietary Supplements. Vitamin D: Moving Toward Evidence-based Decision Making in Primary Care. 2014. [PubMed abstract]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832-5. [PubMed abstract]
- Medical Research Council Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991;338:131-7. [PubMed abstract]
- Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; folic acid (folacin), final rule
 . Federal Register 1996;61:8797-807.
. Federal Register 1996;61:8797-807.
- Food and Drug Administration. Food labeling: Health claims and label statements; folate and neural tube defects
 . Federal Register 1996;61:8752-81.
. Federal Register 1996;61:8752-81.
- Food and Drug Administration. Food standards: Amendment of Standards of Identity for enriched grain products to require addition of folic acid
 . Federal Register 1996;61:8781-97.
. Federal Register 1996;61:8781-97.
- Centers for Disease Control and Prevention. Spina bifida and anencephaly before and after folic acid mandate--United States, 1995-1996 and 1999-2000. MMWR Morb Mortal Wkly Rep 2004;53:362-5. [PubMed abstract]
- Persad VL, Van den Hof MC, Dube JM, Zimmer P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ 2002;167:241-5.[PubMed abstract]
- Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DE. Association of neural tube defects and folic acid food fortification in Canada. Lancet 2002;360:2047-8. [PubMed abstract]
- De Wals P, Rusen ID, Lee NS, Morin P, Niyonsenga T. Trend in prevalence of neural tube defects in Quebec. Birth Defects Res A Clin Mol Teratol 2003;67:919-23. [PubMed abstract]
- Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: A hypothesis. Cancer Epidemiol Biomarkers Prev 2007;16:1325-9. [PubMed abstract]
- Hirsch S, Sanchez H, Albala C, de la Maza MP, Barrera G, Leiva L, et al. Colon cancer in Chile before and after the start of the flour fortification program with folic acid. Eur J Gastroenterol Hepatol 2009;21:436-9. [PubMed abstract]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol 2005;62:641-5. [PubMed abstract]
- Yetley EA, Rader JI. Modeling the level of fortification and post-fortification assessments: U.S. experience. Nutr Rev 2004;62:S50-9; discussion S60-1. [PubMed abstract]
- Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988-2010. J Nutr 2012;142:886-93. [PubMed abstract]
- Bailey RL, Durazo-Arvizu RA, Carmel R, Green R, Pfeiffer CM, Sempos CT, et al. Modeling a methylmalonic acid-derived change point for serum vitamin B-12 for adults in NHANES. Am J Clin Nutr 2013;98:460-7. [PubMed abstract]
- Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, et al. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr 2011;94:552-61. [PubMed abstract]
- Bailey RL, Mills JL, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, et al. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged > or =60 y in the United States. Am J Clin Nutr 2010;92:383-9. [PubMed abstract]
- Bailey RL, McDowell MA, Dodd KW, Gahche JJ, Dwyer JT, Picciano MF. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1-13 y. Am J Clin Nutr 2010;92:353-8. [PubMed abstract]
- Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr 2010;91:231-7. [PubMed abstract]
- Office of Dietary Supplements, National Institutes of Health. Folate: Dietary Supplement Fact Sheet for Health Professionals. 2012.
- Office of Dietary Supplements, National Institutes of Health. Folato: Hoja Informativa para Consumidores. 2013.
- Office of Dietary Supplements, National Institutes of Health. Folate: Fact Sheet for Consumers. 2013.
- Swanson CA, Zimmermann MB, Skeaff S, Pearce EN, Dwyer JT, Trumbo PR, et al. Summary of an NIH workshop to identify research needs to improve the monitoring of iodine status in the United States and to inform the DRI. J Nutr 2012;142:1175S-85S. [PubMed abstract]
- Swanson CA, Pearce EN. Iodine insufficiency: a global health problem? Adv Nutr 2013;4:533-5. [PubMed abstract]
- Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005-2006 and 2007-2008. Thyroid 2011;21:419-27. [PubMed abstract]
- Gahche JJ, Bailey RL, Mirel LB, Dwyer JT. The prevalence of using iodine-containing supplements is low among reproductive-age women, NHANES 1999-2006. J Nutr 2013;143:872-7. [PubMed abstract]
- Office of Dietary Supplements, National Institutes of Health. Analytical Methods/Reference Materials (AMRM) Dietary Supplements Program Description.
- Office of Dietary Supplements, National Institutes of Health. Iodine: Fact Sheet for Health Professionals. 2014.
- Office of Dietary Supplements, National Institutes of Health. Yodo: Hoja Informativa para Consumidores. 2014.
- Office of Dietary Supplements, National Institutes of Health. Iodine: Fact Sheet for Consumers. 2014.
- Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Biomarkers of Nutrition for Development (BOND) Program. 2014.
- Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Biomarkers of Nutrition for Development (BOND) Program: Meetings. 2011.
- Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, Raghavan R, et al. Biomarkers of nutrition for development--iodine review. J Nutr 2014;144:1322S-42S. [PubMed abstract]
- Fulgoni VL, 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr 2011;141:1847-54. [PubMed abstract]
- Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Dietary supplement use is associated with higher intakes of minerals from food sources. Am J Clin Nutr 2011;94:1376-81. [PubMed abstract]
- Bailey RL, Fulgoni VL, 3rd, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet 2012;112:657-63. [PubMed abstract]
- Bailey RL, Fulgoni VL, 3rd, Keast DR, Lentino CV, Dwyer JT. Do dietary supplements improve micronutrient sufficiency in children and adolescents? J Pediatr 2012;161:837-42. [PubMed abstract]
- Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr 2013;143:486-92. [PubMed abstract]
- Dwyer JT, Bailey RL, Britten P, Carriquiry A, Edge MS, Miller D, et al. Fortification: New findings and implications. Nutr Rev 2014;72:127-41. [PubMed abstract]
- Murphy MM, Spungen JH, Barraj LM, Bailey RL, Dwyer JT. Revising the daily values may affect food fortification and in turn nutrient intake adequacy. J Nutr 2013;143:1999-2006. [PubMed abstract]
- Berner LA, Keast DR, Bailey RL, Dwyer JT. Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents. J Acad Nutr Diet 2014;114:1009-22. [PubMed abstract]
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173:355-61. [PubMed abstract]
- Bailey RL, Gahche JJ, Thomas PR, Dwyer JT. Why US children use dietary supplements. Pediatr Res 2013;74:737-41. [PubMed abstract]
- Sorkin BC, Camp KM, Haggans CJ, Deuster PA, Haverkos L, Maruvada P, et al. Executive summary of NIH workshop on the use and biology of energy drinks: Current knowledge and critical gaps. Nutr Rev 2014;72 Suppl 1:1-8. [PubMed abstract]
- Institute of Medicine. Caffeine in Food and Dietary Supplements--Examining Safety: Workshop Summary
 . Washington DC; 2014.
. Washington DC; 2014.
- Camp KM, Lloyd-Puryear MA, Huntington KL. Nutritional treatment for inborn errors of metabolism: Indications, regulations, and availability of medical foods and dietary supplements using phenylketonuria as an example. Mol Genet Metab 2012;107:3-9. [PubMed abstract]
- Camp KM, Parisi MA, Acosta PB, Berry GT, Bilder DA, Blau N, et al. Phenylketonuria Scientific Review Conference: State of the science and future research needs. Mol Genet Metab 2014;112:87-122. [PubMed abstract]
- Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, et al. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet Med 2014;16:188-200. [PubMed abstract]
- Singh RH, Rohr F, Frazier D, Cunningham A, Mofidi S, Ogata B, Splett PL, Moseley K, Huntington K, Acosta PB, Vockley J, Van Calcar SC. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet Med 2014;16:121-31. [PubMed abstract]
- Camp KM, Lloyd-Puryear MA, Yao L, Groft SC, Parisi MA, Mulberg A, et al. Expanding research to provide an evidence base for nutritional interventions for the management of inborn errors of metabolism. Mol Genet Metab 2013;109:319-28. [PubMed abstract]
- Camp KM, Coates PM, Groft SC, Lloyd-Puryear MA, Parisi MA, Urv TK. Select National Institutes of Health research activities for newborn screening disorders: Creating the evidence-base for past and future treatments. In: Poster presented at the American College of Medical Genetics and Genomics Annual Meeting. Nashville, Tennessee; 2013.
- Camp KM. Grant writing and research applications for metabolic dietitians: Interactive workshop. In: Workshop at 14th Abbott Nutrition Metabolic Conference; 2013 June 28; Nashville, Tennessee; 2013.
- Lloyd-Puryear MA, Camp KM, Berry SA. Pediatricians may play role in nutritional management of patients with inherited metabolic disorders. AAP News 2012;33:11.
- Office of Dietary Supplements, National Institutes of Health. Nutrition and Dietary Supplement Interventions for Inborn Errors of Metabolism (NDSI-IEM). 2013.
- U.S. Department of Agriculture. Food Composition and Methods Development Lab
 .
.
- Chen P, Luthria D, Harrington Pd, Harnly JM. Discrimination among Panax species using spectral fingerprinting. J AOAC Int 2011;94:1411-21. [PubMed abstract]
- Sun J, Chen P. Differentiation of Panax quinquefolius grown in the USA and China using LC/MS-based chromatographic fingerprinting and chemometric approaches. Anal Bioanal Chem 2011;399:1877-89. [PubMed abstract]
- Chen P, Harnly JM, Harrington Pd. Flow injection mass spectroscopic fingerprinting and multivariate analysis for differentiation of three Panax species. J AOAC Int 2011;94:90-9. [PubMed abstract]
- Zhao Y, Chen P, Lin L-Z, Harnly JM, Yu L, Li Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem 2011;126:1269-77.
- Sun J, Chen P, Lin LZ, Harnly JM. A non-targeted approach to chemical discrimination between green tea dietary supplements and green tea leaves by HPLC/MS. J AOAC Int 2011;94:487-97. [PubMed abstract]
- Lin LZ, Harnly JM. Phenolic component profiles of mustard greens, yu choy, and 15 other brassica vegetables. J Agric Food Chem 2010;58:6850-7. [PubMed abstract]
- Chen P, Harnly J, Lester G. Flow injection mass spectral fingerprints demonstrate chemical differences in Rio Red grapefruit with respect to year, harvest time, and conventional versus organic farming. J Agric Food Chem 2010;58:4545-53. [PubMed abstract]
- LaBudde RA, Harnly JM. Probability of identification: A statistical model for the validation of qualitative botanical identification methods. J AOAC Int 2012;95:273-85. [PubMed abstract]
- Luthria DL, Mukhopadhyay S, Lin LZ, Harnly JM. A comparison of analytical and data preprocessing methods for spectral fingerprinting. Appl Spectrosc 2011;65:250-9. [PubMed abstract]
- Sun J, Chen P. A flow-injection mass spectrometry fingerprinting method for authentication and quality assessment of Scutellaria lateriflora-based dietary supplements. Anal Bioanal Chem 2011;401:1577-84. [PubMed abstract]
- Xie Z, Zhao Y, Chen P, Jing P, Yue J, Yu LL. Chromatographic fingerprint analysis and rutin and quercetin compositions in the leaf and whole-plant samples of di- and tetraploid Gynostemma pentaphyllum. J Agric Food Chem 2011;59:3042-9. [PubMed abstract]
- Chen P, Lin LZ, Harnly JM. Mass spectroscopic fingerprinting method for differentiation between Scutellaria lateriflora and the germander (Teucrium canadense and T. chamaedrys) species. J AOAC Int 2010;93:1148-54. [PubMed abstract]
- Sun J, Xiao Z, Lin LZ, Lester GE, Wang Q, Harnly JM, et al. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS(n.). J Agric Food Chem 2013;61:10960-70. [PubMed abstract]
- National Institutes of Health. Notice of Availability of Administrative Supplements (Revisions) to NCCAM Grants for Validation Studies of Analytical Methods for Natural Products (NOT-OD-11-016).
- Ren J, Mozurkewich EL, Sen A, Vahratian AM, Ferreri TG, Morse AN, et al. Total serum fatty acid analysis by GC-MS: Assay validation and serum sample stability. Curr Pharm Anal 2013;9:331-9. [PubMed abstract]
- Roman MC. Determination of catechins and caffeine in camillia sinensis raw materials, extracts, and dietary supplements by HPLC-uv: Single-laboratory validation. J AOAC Int 2013;96:933-41. [PubMed abstract]
- Brown PN, Chan M, Betz JM. Optimization and single-laboratory validation study of a high-performance liquid chromatography (HPLC) method for the determination of phenolic Echinacea constituents. Anal Bioanal Chem 2010;397:1883-92. [PubMed abstract]
- Brown PN, Chan M, Paley L, Betz JM. Determination of major phenolic compounds in Echinacea spp. raw materials and finished products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation matrix extension. J AOAC Int 2011;94:1400-10. [PubMed abstract]
- Brown PN. Determination of ginsenoside content in Asian and North American ginseng raw materials and finished products by high-performance liquid chromatography: Single-laboratory validation. J AOAC Int 2011;94:1391-9. [PubMed abstract]
- Oles CJ, Trucksess MW. Determination of fumonisin B1 in botanical roots by liquid chromatography with fluorescence detection: Single-laboratory validation. J AOAC Int 2010;93:1155-60. [PubMed abstract]
- Trucksess MW, Fu WS, Oles CJ, White KD. Determination of zearalenone in botanical dietary supplements, soybeans, grains, and grain products by immunoaffinity column cleanup and liquid chromatography: Single-laboratory validation. J AOAC Int 2011;94:589-95. [PubMed abstract]
- Weaver CM, Trucksess MW. Determination of aflatoxins in botanical roots by a modification of AOAC Official Method 991.31: Single-laboratory validation. J AOAC Int 2010;93:184-9. [PubMed abstract]
- Lam VK, Hung RC, Wong EL, Fok JY, Wong YC. Single-laboratory validation of a method for the determination of vitamin D3 in dietary supplements by liquid chromatography with tandem mass spectrometry (LC-MS/MS). J AOAC Int 2014;97:403-8. [PubMed abstract]
- Waysek EH, Schierle J, Duesterloh A, Deshpande J, Austad J. Determination of lycopene in dietary supplements and raw materials by high-performance liquid chromatography: Collaborative study. J AOAC Int 2010;93:499-509. [PubMed abstract]
- Vyas P, O'Kane AA. Determination of vitamin B12 in fortified bovine milk-based infant formula powder, fortified soya-based infant formula powder, vitamin premix, and dietary supplements by surface plasmon resonance: Collaborative study. J AOAC Int 2011;94:1217-26. [PubMed abstract]
- Brown PN, Yu R, Cain T, Huie G, Jin CD, Kababick JN, et al. Determination of ginsenoside content in Panax ginseng C.A. Meyer and Panax quinquefolius L. root materials and finished products by high-performance liquid chromatography with ultraviolet absorbance detection: Interlaboratory study. J AOAC Int 2013;96:12-9. [PubMed abstract]
- AOAC International Guideline Working Group. AOAC INTERNATIONAL guidelines for validation of botanical identification methods. J AOAC Int 2012;95:268-72. [PubMed abstract]
- Phillips MM, Rimmer CA, Wood LJ, Lippa KA, Sharpless KE, Duewer DL, et al. Dietary supplement laboratory quality assurance program: the first five exercises. J AOAC Int 2011;94:803-14. [PubMed abstract]
- Rimmer CA, Sharpless KE, Wise SA, Betz JM, Coates PM. Standard reference materials for dietary supplement analysis. Anal Bioanal Chem 2013;405:4337-44. [PubMed abstract]
- Sander LC, Bedner M, Duewer DL, Lippa KA, Phillips MM, Phinney KW, et al. The development and implementation of quality assurance programs to support nutritional measurements. Anal Bioanal Chem 2013;405:4437-41. [PubMed abstract]
- Wood LJ, Sharpless KE, Pichon M, Porter BJ, Yen JH, Ehling S. Characterization of three berry standard reference materials for nutrients. J Agric Food Chem 2011;59:7246-52. [PubMed abstract]
- Phillips MM, Case RJ, Rimmer CA, Sander LC, Sharpless KE, Wise SA, et al. Determination of organic acids in Vaccinium berry standard reference materials. Anal Bioanal Chem 2010;398:425-34. [PubMed abstract]
- Lowenthal MS, Phillips MM, Rimmer CA, Rudnick PA, Simon-Manso Y, Stein SE, et al. Developing qualitative LC-MS methods for characterization of Vaccinium berry standard reference materials. Anal Bioanal Chem 2013;405:4451-65. [PubMed abstract]
- Schantz MM, Sander LC, Sharpless KE, Wise SA, Yen JH, NguyenPho A, et al. Development of botanical and fish oil standard reference materials for fatty acids. Anal Bioanal Chem 2013;405:4531-8. [PubMed abstract]
- MacCrehan WA, White CM. Simplified ultrasonically- and microwave-assisted solvent extractions for the determination of ginsenosides in powdered Panax ginseng rhizomes using liquid chromatography with UV absorbance or electrospray mass spectrometric detection. Anal Bioanal Chem 2013;405:4511-22. [PubMed abstract]
- Bedner M, Duewer DL. Dynamic calibration approach for determining catechins and gallic acid in green tea using LC-ESI/MS. Anal Chem 2011;83:6169-76. [PubMed abstract]
- Bedner M, Sander LC, Sharpless KE. An LC-ESI/MS method for determining theanine in green tea dietary supplements. Anal Bioanal Chem 2010;397:1773-7. [PubMed abstract]
- Sharpless KE, Lindstrom RM, Nelson BC, Phinney KW, Rimmer CA, Sander LC, et al. Preparation and characterization of standard reference material 1849 infant/adult nutritional formula. J AOAC Int 2010;93:1262-74. [PubMed absract]
- Phinney KW, Rimmer CA, Thomas JB, Sander LC, Sharpless KE, Wise SA. Isotope dilution liquid chromatography--mass spectrometry methods for fat- and water-soluble vitamins in nutritional formulations. Anal Chem 2011;83:92-8. [PubMed abstract]
- Murphy KE, Vetter TW. Recognizing and overcoming analytical error in the use of ICP-MS for the determination of cadmium in breakfast cereal and dietary supplements. Anal Bioanal Chem 2013;405:4579-88. [PubMed abstract]
- Wood LJ, Lippa KA, Phillips MM, Rimmer CA, Heckert NA, Leigh SD, et al. Breakfast cereal sampling study for nutritional elements. Anal Bioanal Chem 2013;405:4569-78. [PubMed abstract]
- Thomas JB, Sharpless KE, Yen JH, Rimmer CA. Determination of fat-soluble vitamins and carotenoids in standard reference material 3280 multivitamin/multielement tablets by liquid chromatography with absorbance detection. J AOAC Int 2011;94:815-22. [PubMed abstract]
- Sander LC, Sharpless KE, Wise SA, Nelson BC, Phinney KW, Porter BJ, et al. Certification of vitamins and carotenoids in SRM 3280 multivitamin/multielement tablets. Anal Chem 2011;83:99-108. [PubMed abstract]
- Huang M, Winters D. Application of ultra-performance liquid chromatography/tandem mass spectrometry for the measurement of vitamin D in foods and nutritional supplements. J AOAC Int 2011;94:211-23. [PubMed abstract]
- Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem 2012;84:956-62. [PubMed abstract]
- Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr 2008;88:511s-2s. [PubMed abstract]
- Bedner M, Phinney KW. Development and comparison of three liquid chromatography-atmospheric pressure chemical ionization/mass spectrometry methods for determining vitamin D metabolites in human serum. J Chromatogr A 2012;1240:132-9. [PubMed abstract]
- Phillips MM. Analytical approaches to determination of total choline in foods and dietary supplements. Anal Bioanal Chem 2012;403:2103-12. [PubMed abstract]
- Cimino MT. Successful isolation and PCR amplification of DNA from National Institute of Standards and Technology herbal dietary supplement standard reference material powders and extracts. Planta Med 2010;76:495-7. [PubMed abstract]
- Upton R, Graff A, Jolliffe G, Langer R, Williamson E. American Herbal Pharmacopeia: Botanical Pharmacognosy: Microscopic Characterization of Botanical Medicines. Boca Raton, FL: CRC Press; 2011.
- Lin LZ, Sun J, Chen P, Harnly J. UHPLC-PDA-ESI/HRMS/MS(n) analysis of anthocyanins, flavonol glycosides, and hydroxycinnamic acid derivatives in red mustard greens (Brassica juncea Coss variety). J Agric Food Chem 2011;59:12059-72. [PubMed abstract]
- Byrdwell WC. "Dilute-and-shoot" triple parallel mass spectrometry method for analysis of vitamin D and triacylglycerols in dietary supplements. Anal Bioanal Chem 2011;401:3317-34. [PubMed abstract]
- Sun J, Chen P. Chromatographic fingerprint analysis of yohimbe bark and related dietary supplements using UHPLC/UV/MS. J Pharm Biomed Anal 2012;61:142-9. [PubMed abstract]
- Lin LZ, Harnly J, Zhang RW, Fan XE, Chen HJ. Quantitation of the hydroxycinnamic acid derivatives and the glycosides of flavonols and flavones by UV absorbance after identification by LC-MS. J Agric Food Chem 2012;60:544-53. [PubMed abstract]
- Lin LZ, Harnly JM. Quantitation of flavanols, proanthocyanidins, isoflavones, flavanones, dihydrochalcones, stilbenes, benzoic acid derivatives using ultraviolet absorbance after identification by liquid chromatography-mass spectrometry. J Agric Food Chem 2012;60:5832-40. [PubMed abstract]
- Mahoney N, Molyneux RJ. Rapid analytical method for the determination of aflatoxins in plant-derived dietary supplement and cosmetic oils. J Agric Food Chem 2010;58:4065-70. [PubMed abstract]
- Iha MH, Trucksess MW. Aflatoxins and ochratoxin A in tea prepared from naturally contaminated powdered ginger. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2010;27:1142-7. [PubMed abstract]
- Colegate SM, Gardner DR, Joy RJ, Betz JM, Panter KE. Dehydropyrrolizidine alkaloids, including monoesters with an unusual esterifying acid, from cultivated Crotalaria juncea (Sunn Hemp cv. 'Tropic Sun'). J Agric Food Chem 2012;60:3541-50. [PubMed abstract]
- Williams MT, Warnock BJ, Betz JM, Beck JJ, Gardner DR, Lee ST, et al. Detection of high levels of pyrrolizidine-N-oxides in the endangered plant Cryptantha crassipes (Terlingua Creek cat's-eye) using HPLC-ESI-MS. Phytochem Anal 2011;22:532-40. [PubMed abstract]
- Hayward DG, Wong JW, Zhang K, Chang J, Shi F, Banerjee K, et al. Multiresidue pesticide analysis in ginseng and spinach by nontargeted and targeted screening procedures. J AOAC Int 2011;94:1741-51. [PubMed abstract]
- Wong JW, Zhang K, Tech K, Hayward DG, Krynitsky AJ, Cassias I, et al. Multiresidue pesticide analysis of ginseng powders using acetonitrile- or acetone-based extraction, solid-phase extraction cleanup, and gas chromatography-mass spectrometry/selective ion monitoring (GC-MS/SIM) or -tandem mass spectrometry (GC-MS/MS). J Agric Food Chem 2010;58:5884-96. [PubMed abstract]
- Zhang K, Wong JW, Yang P, Hayward DG, Sakuma T, Zou Y, et al. Protocol for an electrospray ionization tandem mass spectral product ion library: Development and application for identification of 240 pesticides in foods. Anal Chem 2012;84:5677-84. [PubMed abstract]
- Office of Dietary Supplements, National Institutes of Health. Dietary Supplement Ingredient Database. 2014.
- Saldanha LG, Dwyer JT, Holden JM, Ireland JD, Andrews KW, Bailey RL, et al. A structured vocabulary for indexing dietary supplements in databases in the United States. J Food Compost Anal 2012;25:226-33. [PubMed abstract]
- Bailey R, Saldanha LG, Dwyer J, Gahche J. Caffeine in energy drinks and dietary supplements in the US. Am J Clin Nutr 2015;in press.
- Dwyer JT, Saldanha LG, Bailen RA, Bailey RL, Costello RB, Betz JM, et al. A free new dietary supplement label database for registered dietitian nutritionists. J Acad Nutr Diet 2014;114:1512-7. [PubMed abstract]
- Office of Dietary Supplements, National Institutes of Health. Computer Access to Research on Dietary Supplements (CARDS) Database. 2014.
- Regan KS, Wambogo EA, Haggans CJ. NIH and USDA funding of dietary supplement research, 1999-2007. J Nutr 2011;141:1-3. [PubMed abstract]
- Office of Dietary Supplements, National Institutes of Health. PubMed Dietary Supplement Subset.
- Office of Dietary Supplements, National Institutes of Health. Evidence-Based Review Program.
- Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, et al. Safety of Probiotics to Reduce Risk and Prevent or Treat Disease
 . Evidence Report/Technology Assessment No. 200. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2007-10062-I.) AHRQ Publication No. 11-E007. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
. Evidence Report/Technology Assessment No. 200. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2007-10062-I.) AHRQ Publication No. 11-E007. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- Trikalinos TA, Lee J, Moorthy D, Yu WW, Lau J, Lichtenstein AH, et al. Effects of Eicosapentanoic Acid and Docosahexanoic Acid on Mortality Across Diverse Settings: Systematic Review and Meta-Analysis of Randomized Trials and Prospective Cohorts
 : Nutritional Research Series, Vol. 4. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
: Nutritional Research Series, Vol. 4. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- Trikalinos TA, Moorthy D, Chung M, Yu WW, Lee J, Lichtenstein AH, et al. Comparison of Translational Patterns in Two Nutrient-Disease Associations
 : Nutritional Research Series, Vol. 5. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
: Nutritional Research Series, Vol. 5. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- Moorthy D, Chung M, Lee J, Yu WW, Lau J, Trikalinos TA. Concordance Between the Findings of Epidemiological Studies and Randomized Trials in Nutrition: An Empirical Evaluation and Citation Analysis: Nutritional Research Series, Vol. 6. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- Shekelle PG, Newberry SJ, Wu H, Suttorp M, Motala A, Lim YW, et al. Identifying Signals for Updating Systematic Reviews: A Comparison of Two Methods
 . Methods Research Report. AHRQ Publication No. 11-EHC042-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
. Methods Research Report. AHRQ Publication No. 11-EHC042-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
- Chung M, Balk EM, Ip S, Lee J, Terasawa T, Raman G, et al. Systematic review to support the development of nutrient reference intake values: Challenges and solutions. Am J Clin Nutr 2010;92:273-6. [PubMed abstract]
- Chung M, Newberry SJ, Ansari MT, Yu WW, Wu H, Lee J, et al. Two methods provide similar signals for the need to update systematic reviews. J Clin Epidemiol 2012;65:660-8. [PubMed abstract]
- Brannon PM, Taylor CL, Coates PM. Use and applications of systematic reviews in public health nutrition. Annu Rev Nutr 2014;34:401-19. [PubMed abstract]
- Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012;307:1959-69. [PubMed abstract]
- Uniformed Services University of the Health Sciences (USUHS). Human Performance Resource Center
 .
.
- Human Performance Resource Center, Uniformed Services University of the Health Sciences (USUHS). Operation Supplement Safety
 .
.
- Institute of Medicine. Use of Dietary Supplements by Military Personnel
 . Washington, DC: The National Academies Press; 2008.
. Washington, DC: The National Academies Press; 2008.
- Yetley EA, Brule D, Cheney MC, Davis CD, Esslinger KA, Fischer PW, et al. Dietary reference intakes for vitamin D: Justification for a review of the 1997 values. Am J Clin Nutr 2009;89:719-27. [PubMed abstract]
- Office of Disease Prevention and Health Promotion, Department of Health and Human Services. health.gov
 . 2014.
. 2014.
- Office of Dietary Supplements, National Institutes of Health. Home Page. 2014.
- Office of Dietary Supplements, National Institutes of Health. Dietary Supplement Fact Sheets.
- Office of Dietary Supplements, National Institutes of Health. Multivitamin/Mineral Supplements.
- Office of Dietary Supplements, National Institutes of Health. Dietary Supplements for Weight Loss. 2014.
- Office of Dietary Supplements, National Institutes of Health. ODS Newsletters.
- Office of Dietary Supplements, National Institutes of Health. My Dietary Supplements (MyDS) Mobile App.
- Office of Dietary Supplements, National Institutes of Health. Vitamin D Initiative.
- Office of Dietary Supplements, National Institutes of Health. ODS Vitamin D Standardization Program.
- Office of Dietary Supplements, National Institutes of Health. Información para el Consumidor.
- Office of Dietary Supplements, National Institutes of Health. Frequently Asked Questions (FAQ).
- Office of Dietary Supplements, National Institutes of Health. Staff Presentations. 2014.
Appendix
Strategic Plan Goals Addressed by Ongoing and New ODS Programs
| ODS Programs and Activities |
Strategic Plan Goals Addressed |
A. Support for Research and Training
- Research and Training Portfolio
- Botanical Research Centers
- Mary Frances Picciano Dietary Supplements Research Practicum |
Goals 1, 2, and 3
Goals 2 and 4
Goals 1, 2, 3, and 4 |
B. Programs Focused on Specific Nutrients and Nutrition/Supplement Interventions
- Vitamin D Initiative
- Population Studies of Folate and Vitamin B12 Status in the United States
- Iodine Initiative
- Evaluating Dietary Supplement Use in the United States
- Nutrition and Dietary Supplement Interventions for Inborn Errors of Metabolism |
Goals 1, 2, and 3
Goal 1
Goal 1
Goals 1, 2, and 4
Goals 1, 2, and 3 |
C. Research Tools
- Analytical Methods and Reference Materials Program
- Dietary Supplement Databases
- Evidence-Based Review Program |
Goals 1, 2, 3, and 4
Goal 1
Goal 1 |
D. Collaborations with other Federal Agencies
- Federal Working Group on Dietary Supplements
- ODS Collaborations with U.S. Department of Agriculture
- Department of Defense–ODS Nutrition Collaboration
- Dietary Reference Intake (DRI) Initiative |
Goals 1 and 4
Goals 1, 2, 3, and 4
Goals 1, 2, and 4
Goals 1 and 4 |
E. Communications
- Communications Program |
Goal 4 |